Introduction
Methodology
Chemical Composition Analysis of Inorganic Construction Wastes
Mix Design of the Recycled Cement
Result and Discussion
Conclusion
Introduction
In the late 20th century, some of the by-products generated in the thoughtless resource utilization in a variety of industrial fields, is committing an error that the living environment damage and Earth’s atmospheric environment pollution. Among these error factors, construction waste is increased by depending on the aging of the buildings. Also, the increase of environmental pollution and shortage of landfill and wasting of effective resources has brought a variety of adverse effects [1, 2].
In the construction material field, eco-friendly materials are actively developed to reduce resources as input and wastes as by-product. However, the focus of the existing studies on recycling of construction wastes, which have been conducted in the construction material industry, has been concentrated on waste concrete [3, 4]. Since the waste concrete is highly available to improve recycling rates, recycled aggregate and cementitious powder have been highlighted. On the other hand, only a few studies dealt with the secondary products using inorganic construction wastes like waste tile, waste cement blocks [5, 6].
The purpose of this study is to analyse the mineral’s creation of recycled cement as a part of development of recycled cement utilizing inorganic wastes.
For this purpose, the inorganic construction waste that is generated in domestic was collected and combined. In addition, produced the clinker of recycled cement using an electric furnace in a laboratory scale. Finally, the optical microscopy and XRD analysis were carried out in order to qualitatively analyze the creation confirmation of the clinker minerals.
Methodology
Chemical Composition Analysis of Inorganic Construction Wastes
For the development of recycled cement selected to collect a variety of inorganic construction waste in domestic generation and analyzed each chemical component of inorganic construction waste using the XRF (X-ray fluorescence). In order to develop high value recycled cement by using diverse inorganic construction wastes, chemical factors related to cement manufacture are to be considered beforehand. As mentioned above, four main chemical components of cement are chemically combined at about 1450℃ in a kiln, which results in new solid minerals. These new minerals are marked C3S (3CaO․SiO2), β-C2S (2CaO․SiO2), C3A (3CaO ·Al2O3), and C4AF (4CaO·Al2O3· Fe2O3) respectively. Table 1 shows chemical composition analysis of inorganic construction waste [7, 8]. The major minerals in the clinker is made up such as C3S (Alite), C2S (Belite), C3A (Aluminate), C4AF (Ferrite) and the characteristics of cement is made up the depends on these differences of the content [9]. The present study considered types and production trends of recyclable construction wastes and analyzed the previous studies on the development of recycled cement using such construction wastes. Based on the analysis, recyclable inorganic construction wastes were selected, and real waste was collected and analyzed. Chemical composition of each inorganic construction waste was analyzed by using an XRF instrument. The chemical composition of the ordinary commercial cement was taken as the baseline. After inorganic construction wastes collected were mixed, they were fired by using Bogue formula. The mineral components of clinker, which was generated from the firing process, was predicted and analyzed [10, 11, 12].
Table 1. Chemical Composition Analysis of Inorganic Construction Wastes
As waste gypsum board and ceiling materials turned out to contain a large amount of calcium oxide., they could substitute limestone, which is a key component of cement. If such inorganic construction wastes are adequately mixed, various types of Portland cement other than the ordinary one could be developed. These major minerals of clinker can be predicted by Bogue formula. Bogue formula was showed in the Table 2 [13].
Table 2. Bogue formula
Mix Design of the Recycled Cement
Table 3 shows the mineral composition ratio of Portland cement (TYPE 1~5) that are defined by the ASTM (American Society for Testing and Materials). The Bogue formula typically calculates the percentage of each cement mineral as follows: C3S (55 wt%), C2S (10 wt%), C3A (10 wt%) and C4AF (10 wt%) [14]. In this study, inorganic construction waste and limestone combined on the basis of the OPC (TYPE 1).
Table 3. The proportion of cement minerals of ASTM standard
| Portland | C3S (wt%) | C2S (wt%) | C3A (wt%) | C4AF (wt%) |
| TYPE 1 | 50 | 25 | 12 | 8 |
| TYPE 2 | 45 | 30 | 7 | 12 |
| TYPE 3 | 60 | 15 | 10 | 8 |
| TYPE 4 | 25 | 50 | 5 | 12 |
| TYPE 5 | 40 | 40 | 4 | 10 |
This study combined inorganic construction wastes by focusing on calcium oxide (CaO) and silicon dioxide (C2S), which are closely related to cement strength and are major influential factors in generating calcium silicate compounds like C3S and C2S. As the goal of this study was develop eco-friendly recycled cement by using inorganic construction wastes, the theoretical combination set the ratios of limestone to 75, 85% to see how much limestone containing much CaCO3 could be theoretically reduced and thus reduce the CO2 emission caused by decarboxylation. In this study, inorganic construction waste and limestone combined on the basis of the OPC as shown in Table 4.
Table 4. Proposed material compositions using inorganic construction wastes
| No | Construction Waste | CASE 1 | CASE 2 |
| 1 | Waste Tile | 4.1 | 4.8 |
| 2 | Waste Cement block | 0.7 | 0.3 |
| 3 | Waste Ceiling material | 17.5 | 7.2 |
| 4 | Limestone | 75.0 | 85.0 |
| 5 | Electric furnace slag | 2.7 | 2.7 |
| Total | 100.0 | 100.0 |
In comparison with limestone (natural resource), as mentioned above, waste ceiling material and waste gypsum board contained a similar level of CaO.
However, they contained much more sulfur trioxide (SO3) components than other inorganic construction wastes. This had to be considered as a variable when mineral composition was predicted by using the Bogue formula. Accordingly, the substitution of limestone by waste ceiling material and waste gypsum board turned out to be below expectation. Waste cement block and waste concrete powder contained a small amount of CaO, as demonstrated in the previous studies, but much more SiO2. For this reason, these materials could not be much applied to the theoretical combinations. Among waste tile and waste clay brick, which were expected to have a large amount of silicate materials, waste tile was applied to combination since it had more CaO and less SiO2. When we make a prediction by using four major minerals (C3S, C2S, C3A, C4AF), no problem occurred without such industrial by-product as electric furnace slag.
The mineral composition of clinker, which would be produced by a real calcination process using the mixing ratios, was predicted and analyzed by the Bogue formula, as presented in Table 5.
Table 5. The result of clinker component analysis using the Bogue formula (cement compound).
| Classification | C3S (wt%) | C2S (wt%) | C3A (wt%) | C4AF (wt%) |
| Standard | 52.00 | 24.00 | 9.00 | 9.00 |
| CASE 1 | 48.38 | 20.15 | 7.15 | 14.14 |
| CASE 2 | 51.27 | 22.34 | 8.12 | 14.90 |
Note) Unit: weight percentage (wt%)
As for C3S, the most influential factor for initial strength, the CASE 2 (51.27%) produced the most approximate prediction. The CASE 1(48.38%) did not satisfy the reference value (52%). C2S makes a much larger contribution to long-term strength than initial one and has both low reactivity and low calorie. All of combinations failed to reach the reference value of C2S (CASE 1 : 20.15%, CASE 2 : 22.34%). Also, the reference value of C3A, which has high reactivity and very high calorie, was not satisfied in all of combinations (CASE 1 : 7.15%, CASE 2 : 8.12%). On the other hand, C4AF was predicted to exceed its reference value. Generally, the CASE 2 turned out to be the idealist combination for the Ordinary Portland Cement that was the standard, since it predicted and analyzed the highest levels of C3S and C2S, which affected the initial and long-term strengths, respectively.
Result and Discussion
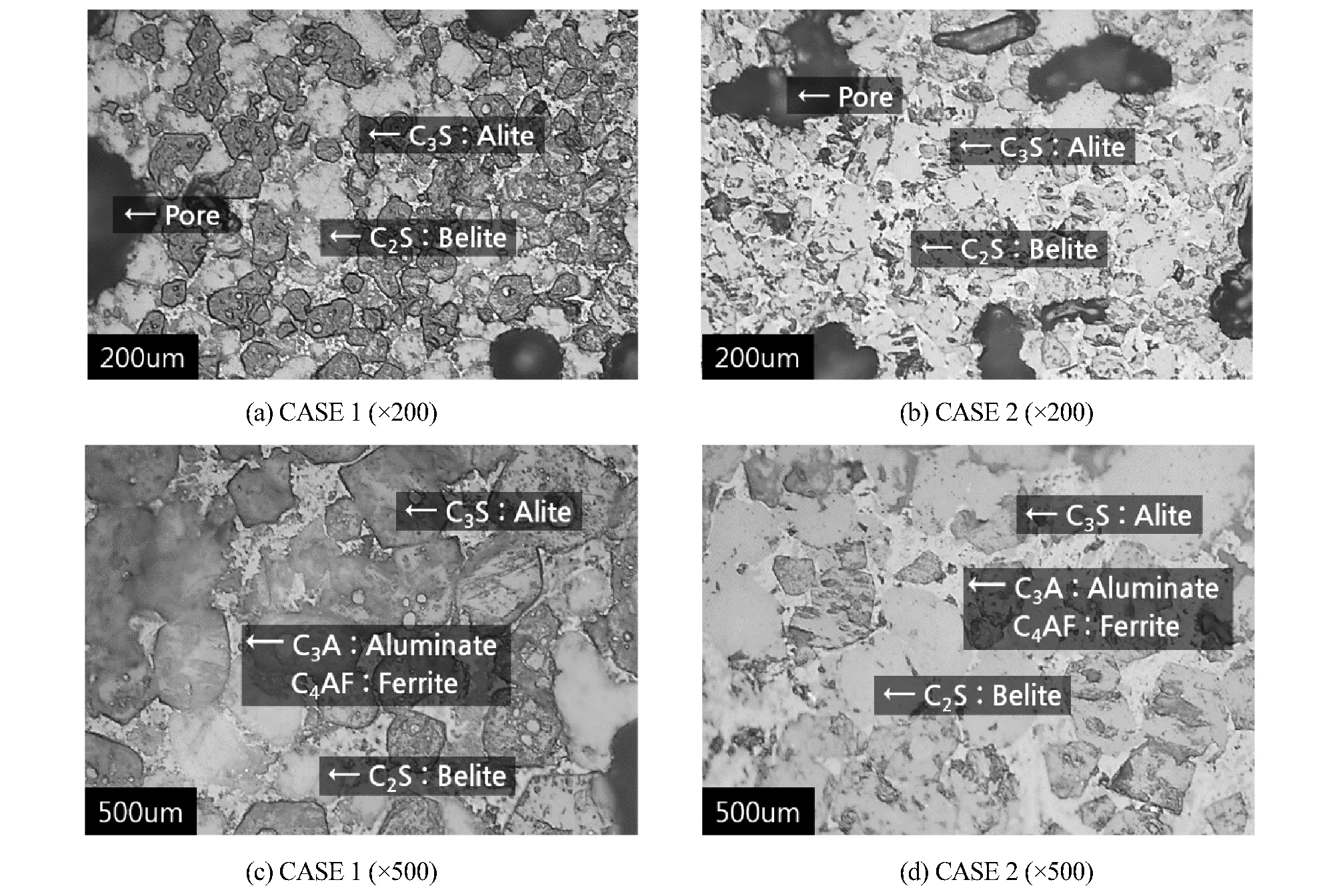
Figure 1 is a photograph using an optical microscope after polishing a cross section of a recycled cement clinker. CASE 1(a) and CASE 2(b) are photographs that is taken by zoom 200 times of cross section of clinker, it was confirmed that the C3S and C2S is generated uniformly distributed. Also, in the CASE 1(c) and CASE 2(d) (zoom 500 times), C3S and C2S as well as C3A and C4AF could be confirmed. Large crystals angular of 20~50 ㎛ size is C3S and crystals in which the round shape of 15~20 ㎛ size is C2S. In addition, C3A and C4AF is fine particle of between C3S and C2S. That is called interstice matter (Puertas, 2008).
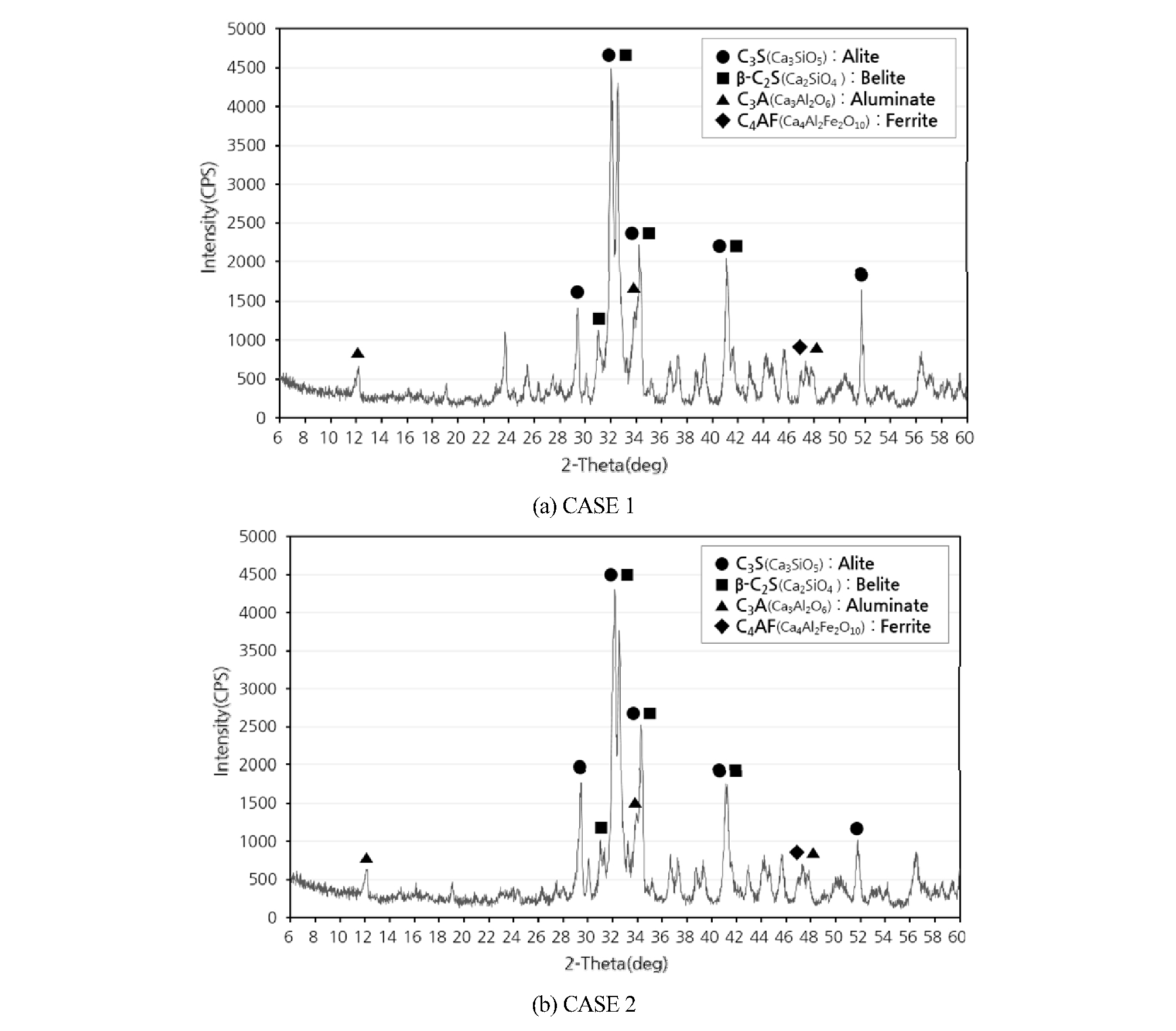
Recycled cement that is manufactured in this study was analyzed mineral component of clinker by the XRD analysis, and its results are shown in Figure 2. According to theoretical study, all the peaks that is indicating main minerals of cement is shown at between 29 and 33 degree (Paul E, 2016). Similarly, in this study, as with the theoretical study, both graphs show a peak of main mineral of cement such as C3S, C2S, C3A and C4AF. However, XRD analysis quantitatively to analyze the amount of the mineral is limited.
This study dealt with the development of recycled cement through 2 CASES. As pointed out in the previous studies, inorganic construction wastes contained main chemical components of cement and thus could be candidate substitutes of raw materials of cement. However, each inorganic construction waste is produced at different time and places and by various methods. Accordingly, they did not show a uniform chemical composition but contained many impurities. While recycled cement is really fabricated, such impurities will become an obstacle to uniform quality of product and may frustrate quality management. To solve this problem, the management system for all the related stakeholders in the whole procedure from construction sites, which discharge wastes, to collection yards and transport stage needs to be improved. Such improvement can include a new manual and incentive system so that construction wastes could be effectively classified and disposed based on their properties. If construction wastes are well classified and easily collected, more predictable and uniform cement substitutes will be secured.
Conclusion
This study analysed whether the inorganic construction waste is used as raw material for cement using the optical microscope and XRD analysis. Especially, through the microscope analysis it was confirmed that the C3S and C2S is generated uniformly distributed. And, it could be confirmed that is peak of main mineral of cement such as C3S, C2S, C3A and C4AF by the XRD analysis. The inorganic construction wastes were collected based on the Ordinary Portland Cement. Their chemical components were put into the Bogue formula to predict the generation of minerals after clinker calcination. Consequently, it turned out that, if limestone accounted for over 85%, a new type of cement with the same quality level as the Portland Cement could be developed. As for waste ceiling material and waste gypsum board, if sulfur trioxide (SO3) contained in a large amount could be removed, these wastes will substitute limestone for manufacturing recycled cement.
However, the composition of the cement clinker minerals has been determined by various factors such as combinations and thermal properties of raw materials. Based on this study, there is a need for a variety of study such as physical or chemical study as part of the development of the recycled cement utilizing inorganic construction wastes.