Introduction
Fabrication of electrochromic laminated glass specimen
UV cured polymer resin-based high strength electrolyte
Optimizing the sputter disposition of the color-changing layer and ion-storage layer glasses
Fabrication of electrochromic laminated glass specimen
Results of performance evaluation of electrochromic laminated glass
Analysis results of polymer resin-based high strength electrolyte ionic conductivity
Analysis results of the bond strength of electrochromic laminated glass
Results of analyzing the coloration efficiency of electrochromic laminated glass
Analysis results of the optimal properties of electrochromic laminated glass
Conclusion
Introduction
Implementing a zero-energy building program beyond energy reduction requires superior thermal insulation performance as well as the solar control performance on the building envelope. In particular, it is imperative to ensure the solar control performance on the building envelope under climatic conditions characterized by clear seasonal cycles of summer and winter.
One of the most commonly adopted solar control systems in Korea is the installation of rolling blinds or shades on the indoor side of the window controlled by a user on an as-needed basis. The existing solar controllers are mostly designed to reduce glare [1], which is ineffective in saving energy because it is installed on the indoor side and blocks solar radiation after it is infiltrated into the indoor [2]. In recent years, there have been active R&D efforts on smart glasses that enable to control the transmittance on the glass level, which is effective in saving energy as it blocks solar radiation before it is infiltrated into the indoor in addition to providing the anti-glare function [3]. There are several smart glass technologies whose R&D effort has been underway in recent years. First, the electrochromic glass features a wide range of transmittance throughout the entire solar spectrum thanks to its feature of changing colors, activation at a low voltage of 5 V or less [4], and maintenance of the current state without application of electricity if the desired transmittance is reached as long as electricity is supplied when changing transmittance is needed [5].
PDLC (Polymer dispersed liquid crystal) is more economical than any other smart glass with a quick response time of 1 second or less [6]. It can be used as laminated glass as it is easily manufactured in the form of a film and also can be attached to the existing glass in the form of a film, which is a differentiating advantage. Thermochromic and photochromic feature changing transmittance according to temperatures and ambient light, respectively [7]. One of the key advantages of thermochromic and photochromic is that the transmittance can be adjusted without a separate power supply, which is, however, a double-edged sword as the transmittance is likely to be controlled even without the intervention of user [8].
Active R&D on electrochromic among smart glasses is underway at home and abroad for the commercialization of electrochromic glass among smart glasses [9]. The key components of the electrochromic glass include color-changing layer, ion-storage layer and the electrolyte [10]. Active research is underway on various chemical materials for major components of electrochromic glass [11], and this study focuses on the electrochromic laminated glass based on the polymer resin electrolyte.
In material engineering, the inherent material characteristics of electrochromic such as coloration efficiency, ion conductivity, and transmittance are the most important performance factors. However, from an architectural perspective where the electrochromic is one of the building materials, structural stability and durability are just as important as color-changing characteristics.
The use of gel electrolytes in developing electrochromic materials allowed ionic conductivity between the laminated glasses. However, our research team found a problem in using gel electrolytes in manufacturing large-area glasses as a building material, which is their excessive sensitive to small scale impacts due to lack of bond strength. As a result, we often witnesses the occurrence of crevice on the center of the glass and subsequent loss of ionic conduction in manufacturing process and the loss of the electrochromic properties of gel electrolytes due to exfoliation on the color-changing layer and the electrolyte layer in the process of restoring the sagging windows caused by wind load. More importantly, low bond strength makes glasses vulnerable to possible glass fallout and consequential serious safety accidents due to low bond strength.
In order to resolve this low bonding strength issue, this study has developed and evaluated on the performance of electrochromic windows with high bond strength using polymer resin-based electrolytes. The performance evaluation has been conducted on the bond strength, ionic conductivity, coloration efficiency, and the variation of transmittance of the newly developed electrochromic windows to identify their material properties and the applicability as building glasses.
Since there is no commercialized product of electrochromic glass in Korea, there is no comparison group to explain the advantages and disadvantages of the material developed in this study. The result is optimized performance by changing the material and process conditions of the material. To compare the performance, the performance was compared based on the literature and previous studies
Fabrication of electrochromic laminated glass specimen
UV cured polymer resin-based high strength electrolyte
The electrolyte acts as a pathway for oxidation, reduction and the ion transfer between the color-changing layer and the ion-storage layer of the electrochromic glass. The electrolyte developed in this study has used lithium salt and polycarbonate (PC) as the organic solvent to liquefy lithium as it comes in high purity powder form. The initiator has also been used to allow ultraviolet (UV) curing when bonding the two sheets of glass, including the color-changing layer and the ion-storage layer. In order to enhance the bond strength, a primary purpose of developing the electrolyte, the polycarbonate has been blended with polymer resin to produce the electrolyte for high strength electrochromic laminated glasses.
Optimizing the sputter disposition of the color-changing layer and ion-storage layer glasses
To manufacture electrochromic color-changing layer and ion-storage layer requires sputter deposition on tungsten oxide (WO3) and nickel oxide (NiO) targets on the glass coated with a transparent conductive object (TCO) in vacuum conditions. This study has fabricated the color-changing layer and the ion-storage layer by sputter deposition of WO3 and NiO using indium tin oxide (ITO) as a transparent conductive film glass. Here, sputter disposition refers to the a process of sputtering or dispersing material from a target by connecting a metal to the negative pole and discharging in vacuum or decompressed condition and then depositing it onto a solid substrate such as glass.
In the sputtering process, the vacuum condition affects the extent of variation in transmittance of the color- changing layer and the ion-storage layer. The variable that most affects the extent of variation in transmittance in sputtering vacuum conditions is the mixture ratio between argon gas and oxygen. Table 1 shows the transmittance, extent of variation of transmittance, and the optical density (OD) associated with the argon-oxygen mixture ratio in depositing WO3, a color-changing layer. Here, the optical density (OD) is calculated by the equation (1), where indicates the transmittance of the bleached state, and indicates the transmittance of the cleared state. The optical density (OD) indicates the ratio of color transmittance in bleached state over in cleared state and is used to calculate coloration efficiency (CE) in the next chapter.
Table 1. Transmittance variation and optical density of WO3 spattered glass by argon-oxygen ratio
| Ar : O2 | Transmittance | dT(%) | dOD | |
| Colored | Bleached | |||
| 50 : 50 | 56.2 | 76.7 | 20.6 | 0.135 |
| 60 : 40 | 37.9 | 70.8 | 33.0 | 0.272 |
| 70 : 30 | 12.3 | 77.5 | 65.2 | 0.799 |
| 80 : 20 | 75.0 | 79.05 | 4.1 | 0.023 |
| $$\triangle OD=\log\frac{T_b}{T_c}$$ | (1) |
The analysis results in Table 1 have found that the variation in transmittance and the optical density gradually increase as the relative portion of argon gas increases with the mixture ratio between argon and oxygen in depositing WO3 turning from 50:50 to 70:30 although they sharply decrease as the ratio reaches 80:20 and on. Based these findings, the color-changing layer glass deposited with WO3 has been fabricated under the condition of the argon-oxygen mixture ratio of 70:30.
Table 2 shows the variation in transmittance and optical density (OD) by sputtering condition in fabricating the ion-storage layer glass fabrication, which indicates that the variation in transmittance and the optical density gradually increase as the relative ratio of argon gas increases. Accordingly, the ion-storage layer glass deposited with NiO has been fabricated under the condition of argon-oxygen ratio of 90:10.
Table 2. Transmittance variation and optical density of NiO spattered glass by argon-oxygen ratio
| Ar : O2 | Transmittance | dT(%) | dOD | |
| Colored | Bleached | |||
| 60 : 40 | 69.4 | 64.2 | 5.2 | 0.034 |
| 70 : 30 | 69.8 | 60.8 | 9.0 | 0.059 |
| 80 : 20 | 70.5 | 60.9 | 7.6 | 0.063 |
| 90 : 10 | 72.6 | 56.7 | 15.9 | 0.107 |
Fabrication of electrochromic laminated glass specimen
Earlier, the study covered how to fabricate high-strength electrolyte using polymer resin and the optimized argon-oxygen ratio to fabricate the color-changing layer (WO3) and the ion-storage layer (NiO). This chapter describes how to fabricate electrochromic laminated glass specimens using electrolyte, color-changing layer (WO3) glass, and ion-storage layer (NiO) glass.
The fabrication process of electrochromic laminated glass is shown in Table 3. First, a piece of color-changing layer and a piece of ion-storage layer glass with the dimension of 70 mm × 70 mm have been prepared. The size of the specimen has been set to maximum measurable by the measuring instrument in analyzing the bond strength, color-changing characteristics, and the optical properties to be described in the next chapter. Due to a need of space of 1mm height to build an electrolyte layer between the color-changing layer and the ion-storage layer glass, a 1mm-thick structural double-sided tape has been attached to the corners to prepare a space between the laminated glasses with 5 mm crevice to inject electrolyte when attaching the structural double-sided tape. Next, after bonding the color-changing layer and ion-storage layer glasses, we injected the electrolyte between two pieces of glass using a dropping pipette, filled the groove with a sealing agent, and then left them cured for a predetermined period of time using an ultraviolet lamp to fabricate an electrochromic laminated glass specimen. Next chapter will describe the ionic conductivity, bond strength, coloration efficiency and the optical properties of electrolytes based on the fabricated electrochromic laminated glass specimens.
Table 3. Electrochromic laminated glass manufacturing process
| ➀ WO and NiO coated glass preparation | ➁ Electrolyte injection |
.jpg) | .jpg) |
| ➂ Lamination by UV curing process | ➃ Electrochromic glass specimen |
.jpg) | .jpg) |
Results of performance evaluation of electrochromic laminated glass
Analysis results of polymer resin-based high strength electrolyte ionic conductivity
Electrochromism is the phenomenon where the color of the color-changing layer and the ion-storage layer changes when an electric voltage + or - is applied through repetition of oxidation and reduction as electric charges are transferred by icons included in electrolytes. Electrolytes play a role of transferring charges in the color- changing layer and the ion-storage layer when the ionic conductivity affects the performance of the color-changing glass. The higher the ionic conductivity is, the higher the color-changing rate and the efficiency will be.
To evaluate the ionic conductivity of the polymer resin-based electrolyte developed in this study, electrolytes have been inserted into a fixing frame and ultraviolet (UV) has been inspected to fabricate in a thin film form as shown in Table 4. Electrodes with a diameter of 16mm and an area of 2.011cm2 have been built in front and rear of the fabricated film and then the resistance and ionic conductivity have been evaluated using the electrochemical measuring instrument, Solartron SI 1287 and SI 1255.
Table 4. Electrochromic laminated glass manufacturing process
| ➀ Electrolyte film production | ➁ Measuring cell for ion conductivity |
.jpg) | .jpg) |
Table 5 shows the ion conductivity performance analysis results for the two electrolyte films, producing the values of 2.73 × 10-4 S/cm, 2.41 × 10-4 S/cm with the average ionic conductivity of 2.57 × 10-4 S/cm . In terms of the ionic conductivity performance levels by electrolyte type, the gel electrolyte, PVB film-type electrolyte and the solid electrolyte have produced 3 × 10-4 S/cm, 2 × 10-5 S/cm, and 3 × 10-6 S/cm, respectively. Therefore, the ionic conductivity of the electrolyte developed in this study has been found to be similar to that of the gel-type electrolyte.
Table 5. Transmittance variation and optical density of NiO spattered glass by argon-oxygen ratio
| No | Thickness | Diameter | Area | Resistance | Conductivity |
| 1 | 2.36 mm | 16 mm | 2.011 cm2 | 430.7 Ohm | 2.73 × 10-4 S/cm |
| 2 | 2.09 mm | 16 mm | 2.011 cm2 | 431.1 Ohm | 2.41 × 10-4 S/cm |
Analysis results of the bond strength of electrochromic laminated glass
This study has aimed to develop an electrolyte with high bond strength to enhance the structural stability of electrochromic laminated glasses and to contribute to commercializing it as a building material featuring safety and durability.
Therefore, the most important evaluation element in this study is the bond strength, and the study intends to identify the degree of bond strength compared to that of the existing gel electrolyte. The existing gel electrolyte has been developed by the company S, and the test has been conducted on most frequently used electrolyte in developing the electrochromic laminated glass in Korea with the use of the UV-cured gel electrolyte.
There are no domestic standards to evaluate the bond strength of electrochromic glasses. Thus, this study has conducted the bond strength test in compliance with the KS F 4042 “Polymer Modified Cement Mortar for Maintenance in Concrete Structure”. As shown in Table 6, an epoxy adhesive has been applied to a jig, an electrochromic laminated glass has been attached and then the bond strength has been measured using the universal testing machine (UTM). The test on the electrochromic laminated glass has been conducted without the tape attached to the edges to evaluate the bond strength generated by electrolytes only.
Table 6. Electrochromic laminated glass bonding strength test process
| ➀ Preparation jig with epoxy adhesive | ➁ Electrochromic specimen attached to jig |
.jpg) | .jpg) |
| ➂ Preparation jig with electrochromic specimen | ➃ Bond strength test by UTM equipment |
.jpg) | .jpg) |
Table 7 shows the bonding strength test results. The gel electrolyte is found to be 0.178 Mpa in bond strength while the high strength electrolyte developed in this study is found to be 0.364 Mpa, which is 2.04 times higher bond strength performance that the gel electrolyte. This study has also analyzed the adhesive strength of the developed electrolyte compared to that of the gel electrolyte to evaluate the bond strength and is going to conduct another bond strength test in accordance with the KS L 2004 “Laminated glass”.
Table 7. Result of bonding strength test of each electrolyte
| Division | Bonding strength |
| Gel type electrolyte | 0.178 Mpa |
| High strength electrolyte | 0.364 Mpa |
Results of analyzing the coloration efficiency of electrochromic laminated glass
This chapter compares the coloration efficiency(CE) between the existing gel electrolyte and high strength electrolyte during the cycle of coloration and discoloration of electrochromic glass. This study has used the same glass for the color-changing layer and the ion-storage layer with the adoption of different electrolytes.
Table 8 shows the test procedure to analyze the coloration efficiency (CE) of the electrochromic laminated glass. Obtaining the coloration efficiency (CE) requires simultaneous measurement of both the current density (Q) and the visible light transmittance during the oxidation and reduction process when +/- electric voltage is applied to the electrochromic glass. Therefore, this study has used the measuring instrument that allows the simultaneous measurement of both the current density (Q) and the visible light transmittance retained by the Korea Electronics Technology Institute to obtain the coloration efficiency (CE).
Table 8. Coloration efficiency test process of electrochromic laminated glass
| ➀ Insert specimen into instrument | ➁ Coloration efficiency test equipment |
.jpg) | .jpg) |
The coloration efficiency(CE) is the efficiency of extent of transmittance variation for the charge consumed per unit area when +/- electric voltage is charged with higher extent of transmittance variation with a small amount of energy indicating higher efficiency. The formula (2) represents the equation to obtain the coloration efficiency (CE), where Q is the charge density (Q) and means the optical density.
| $$CE=\frac{\triangle OD}Q$$ | (2) |
Table 9 shows the coloration efficiency (CE) analysis results between the gel electrolyte-based electrochromic laminated glass and the high strength electrochromic laminated glass developed in this study. First, the gel electrolyte-based electrochromic laminated glass shows the charge consumed per unit area of 10.07 μC/cm2 and –10.05 μC/cm2, while the high strength electrochromic laminated glass has charge consumed per unit area of 7.8 μC/cm2 and –7.8 μC/cm2, indicating that the current density (Q) of the gel electrolyte-based glass is higher than that of the high strength glass. Higher current density Q means that a larger amount of lithium ion charges transferred in the electrolyte. The current density (Q) shows a similar tendency to the ionic conductivity with the gel electrolyte found to be higher than the high strength electrolyte.
Table 9. Result of coloration efficiency test of electrochromic lamanated glass
In terms of variations in transmittance, of the gel electrolyte is found to be 51.38% and that of the high strength electrolyte is found to be 57.29%, which is higher than the gel electrolyte. of the gel electrolyte is found to be 0.638 and that of the high strength electrolyte is 0.648, which is also higher than the gel electrolyte.
The coloration efficiency of the gel electrolyte is found to be 63.36 and that of the high strength electrolyte is 83.08, which is higher than that of the gel electrolyte, indicating a higher degree of transmittance with lower current density (Q). Therefore, the polymer resin-based high strength electrolytes can enhance the bond strength as well as the coloration efficiency (Q) more effectively than the existing gel electrolytes. These results are considered to originate from the difference in terms of the type and mixture ratio of the materials comprising the electrolyte although it is not definitive as the manufacturing method of electrolyte materials is yet to be disclosed.
Analysis results of the optimal properties of electrochromic laminated glass
The electrochromic glass is an architectural glass material commonly used for building envelope and allows a solar control. Solar radiation is comprised of ultraviolet, visible and infrared rays according to the spectral ranges, all of which are radiant energy affecting cooling and heating energy and visible rays also affect the lighting energy as well.
This chapter describes analyzing the optical properties of electrochromic glasses that have a direct effect on building energy. First, the optical properties of electrochromic glasses have been analyzed using the spectrometer in accordance with KS L 2514 standard as shown in Table 10.
Table 10. Coloration efficiency test process of electrochromic laminated glass
| ➀ Insert specimen into instrument | ➁ Coloration efficiency test equipment |
.jpg) | .jpg) |
Table 11 shows the solar transmittance, solar reflectance, visible light transmittance, and visible light reflectance as the optical properties analysis results. The solar transmittance that corresponds to the entire range of spectrum (300 nm ~ 2500 nm) with the greatest impact on building energy shows 53.1% of variation range from 61.1% in clear state and 8.0% in bleached state.
Table 11. Optical properties of electrochromic laminated glass
| Optical properties | Bleached | Colored |
| Solar transmittance | 0.611 | 0.080 |
| Solar reflectance | 0.153 | 0.107 |
| Visible transmittance | 0.739 | 0.166 |
| Visible reflectance | 0.119 | 0.076 |
The analysis of the optical properties of electrochromic laminated glasses has found such glasses effective in reducing heating and cooling energy by accepting solar radiation in winter and blocking it in summer thanks to a wide variation of solar and visible light transmittance. In addition, the visible light transmittance is as low as 16.6% in bleached state, which will reduce glare by blocking the solar radiation in the occurrence of discomfort glare and also reduce the lighting energy by controlling the visible light transmittance. Based on the analysis results of the optical properties, the study is going to conduct analysis on the building energy and environment performance using the dynamic building energy simulation.
Based on the results of the optical properties analysis of electrochromic laminated glass, the study has conducted an analysis on the solar heat gain coefficient (SHGC) and visible light transmittance (Tvis) in constructing architectural double glazed glasses.
As an analysis method, the optimal properties data whose spectroscopic analysis has been conducted within the range between 300 nm and 2500 nm on a 5 nm unit have been converted into the average solar transmittance and reflectance and the average visible light transmittance and the reflectance using the Optic 6 program of LBNL. And then, the converted results have been inserted into the LBNL Window 7.4 program and the electrochromic double glazed glass has been constructed using the 6mm transparent glass and 12 mm air layer of a domestic glass manufacturer provided as IGDB to obtain the optimal properties data.
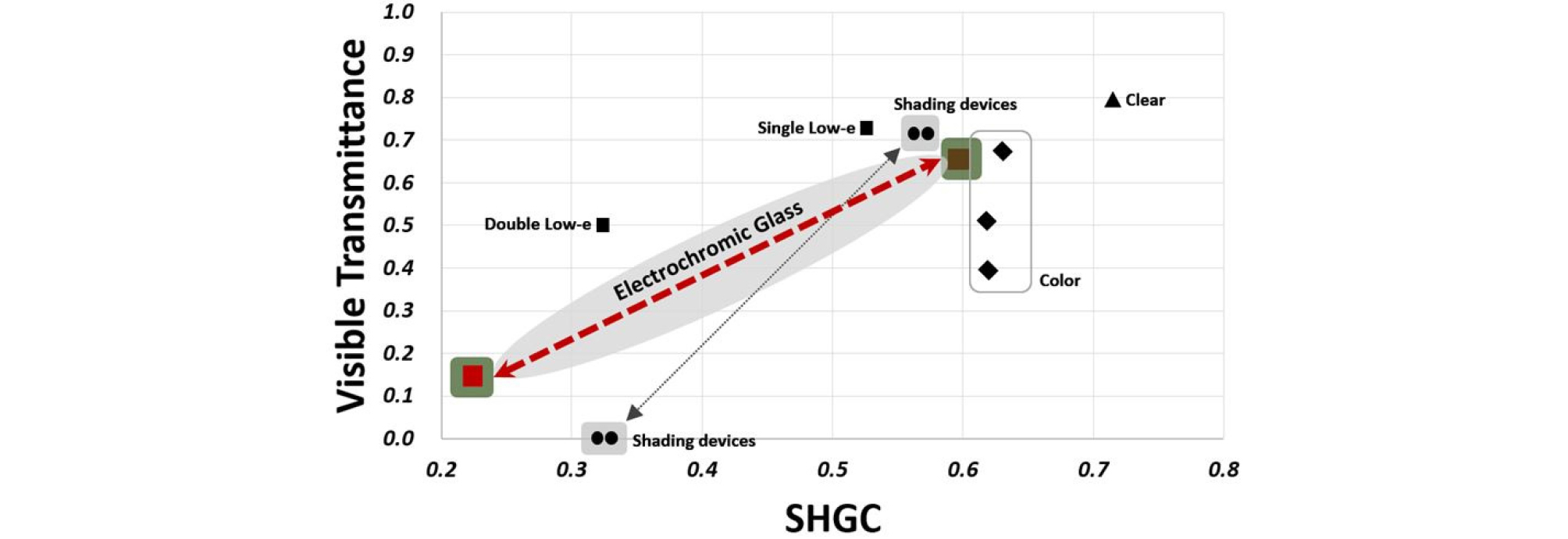
Figure 1 is a graph showing the optical characteristics data of transparent laminated glass, colored glass laminated glass, low-e laminated glass, laminated glass with indoor sunshade, and electrochromic laminated glass using LBNL Window 7.4 program. The environmental conditions were derived at the indoor temperature of 20ºC and the outdoor temperature of 0ºC in accordance with Korean Industrial Standard (KS).
Figure 1 is a graphical representation of the optical properties of transparent double glazed glass, colored glass double glazed glass, Low-e double glazed glass, double glazed glass equipped with indoor shading device, and electrochromic double glazed glass that are available in domestic markets using the LBNL Window 7.4 program.
Figure 1 shows the applicability of the electrochromic laminated glass developed in this study as a reduction technology to reduce building energy. The electrochromic laminated glass has the optical properties comparable to those of the double glazed glass in cleared state and is found to have a lower solar heat gain coefficient than that of the double glazed glass outfitted with an indoor blind or roll shade in bleached state.
These results support the superior performance of the electrochromic laminated glass a in terms of solar controlling function as it features a wide variation in transmittance and lower solar radiation blocking rate than the indoor shading device that is commonly used in commercial office buildings. Weaknesses to be compensated for in the follow-up studies require comparative evaluation of various comparison groups and verification of cycling performance.
Conclusion
This study has fabricated the electrochromic laminated glass using high strength electrolytes and evaluated its performance. The performance evaluation of ionic conductivity of electrochromic laminated glass has found the ionic conductivity of electrolytes unexpectedly high. Contrary to the expectations that the ionic conductivity would be as low as that of PVB(Polyvinyl butyral) or solid electrolytes as it is blended with polymer resin to increase the strength, the rest has found its ionic conductivity similar to that of the gel electrolyte.
The performance evaluation for bond strength has found that the high strength electrolyte is 2.04 times higher than the existing gel electrolyte. This study has evaluated the bond strength based on the test standard for adhesive power, and the follow-up study is going to evaluate the bond strength performance through the “laminated glass” test in accordance with the KS 2004 Standard.
The newly developed high strength electrochromic laminated glass has also found to be more superior to the existing gel electrolyte in coloration efficiency (CE) as well, featuring a wide range of transmittance with less amount of charge than that of the gel electrolyte. Following the analysis of the structural performance and color-changing properties of electrochromic materials, an analysis on optical properties has been conducted to find out the solar transmittance, visible light transmittance and the solar heat gain coefficient that are directly related to building energy. The optical properties analysis results have found 53.1% of the variation range in solar transmittance and 57.3% of the visible light transmittance, indicating that it is effective as solar controlling smart glasses in reducing building energy under domestic climate conditions characterized by clear seasonal distinction between summer and winter.
The comparative analysis between the architectural double glazed glasses built using LBNL Window 7.4 and the existing commercially available laminated glasses, indoor blinds, and roll shades has found that the electrochromic laminated glass has the widest variation in transmittance and the lowest Solar heat gain coefficient in colored state compared to the existing products, which is expected to contribute to reducing building energy and enhancing the lighting environment performance thanks to its highly effective blocking of solar radiation.
This study has produced laboratory level results but made one step closer to the goal of developing the electrochromic laminated glass featuring color-changing characteristics while meeting the structural performance requirements as a building material in the construction field. Although it is difficult to get access to the detailed information on the electrolyte and electrochromic glasses as they are kept confidential, we plan to carry out research and development on related materials while reviewing the performance specifications required from the architectural perspectives. Subsequent research will analyze the applicability as building materials by performing evaluation of building energy performance and daylight performance using electochromic glass optical property data driven by this research.