Introduction
Reinforcement concrete Corrosion -Corrosion by chlorides-
Depassivation mechanism
Corrosion Models
Theoretical formulation
Probabilistic Analysis of Reinforced Concrete Corrosion
Results and Discussion
Conclusion
Introduction
The degradations induced by the external conditions are ordered by defining several classes of exposure for the corrosion risk, depending on the environmental actions and concrete work conditions [1], [2].
Chemical factors are frequently the most important, since the reinforcement concrete can be degraded by reaction (dissolution, swelling, corrosion…) of the reinforcement concrete constituents.
Minimal concrete covers requirements are associated with these classes. Among these classes, there is that corresponding to the corrosion induced by carbonation, which applies to the reinforced concrete exposed to the air and moisture.
Carbon dioxide and chlorides are the most important buildings aggressive agents which may be exposed. They are responsible for one of the main mechanisms of degradation of existing structures.
These phenomena are respectively identified by the corrosion due to chlorides penetration or corrosion by carbonation.
The speed of reinforcement concrete corrosion depends mainly on the chlorides penetration inside the cement matrix. Indeed, the chlorides diffusion through the concrete porous structure is determined by the Water to cement ratio and porosity [3], [4].
A deterministic models proposed Poulsen, Maage and Duracrete fixes the chloride ions migration coefficient and critical chloride ion content, comprise some limits related to the random variation of the input model parameters, and the precise knowledge of these parameters requires a probabilistic approach enable to modeling the uncertainties and analyzing their dispersion effect [5].
In this paper, a probabilistic formulation is applied to reinforcement corrosion phenomenon and statistics regarding chloride penetration depth are investigated by performing a parametric analysis which integrates the influence of chloride ions migration coefficient variability and critical chloride ion content.
Reinforcement concrete Corrosion -Corrosion by chlorides-
Chlorides can come from several sources. They can be cast into the concrete or they can diffuse in from the outside. Chloride cast into concrete can be due to use of seawater in the mix, a contaminated aggregates and chloride set accelerators (calcium chloride CaCl2).
Also, Chlorides can diffuse into concrete due to use of chemicals (structures used for salt storage, brine tanks, aquaria, etc.), sea salt spray, direct seawater wetting and deicing salts; [5].
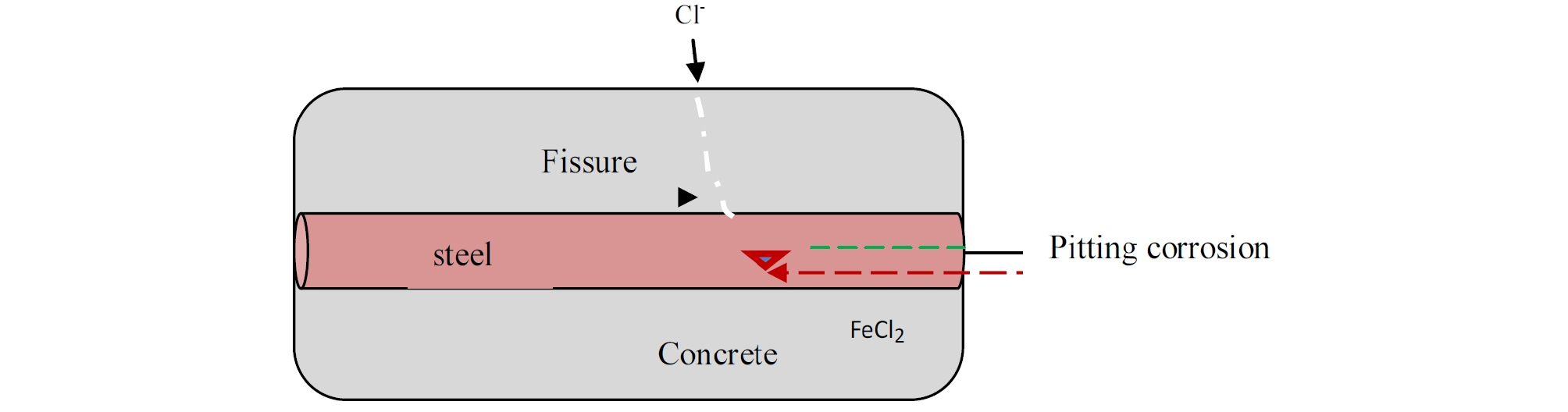
The diffusion of chlorides into concrete as that is the major problem in most parts of the world either due to marine salt spray or use of deicing salts. It is happens in marine conditions where sea water contaminates the original concrete mix and then diffuses into the hardened concrete and reach the armatures.(Figure 1) [6].
The reactions of the Cl- ions with the Fe ++ take place as follows: [7].
| $$\mathrm{Fe}^{++}+\mathrm{Cl}^-\;\rightarrow\;{\mathrm{FeCl}}_2(\mathrm{iron}\;\mathrm{chloride})$$ | (1) |
| $${\mathrm{FeCl}}_2+2\mathrm{OH}^-\rightarrow\;\mathrm{Fe}(\mathrm{OH})_2+2\mathrm{Cl}^-$$ | (2) |
| $$\mathrm{Fe}(\mathrm{OH})_2+{\mathrm O}_2\rightarrow\mathrm{rust}$$ | (3) |
Cl- chloride ions are recycled, which explains the absence of chloride in the rust.
Depassivation mechanism
The chloride ion attacks the passive layer. Chlorides act as catalysts to corrosion. They are not consumed in the process but help to break down the passive layer of oxide on the steel and allow the corrosion process to proceed quickly.
The effective recycling of chloride ions makes chloride attack more difficult to remedy as chlorides are therefore harder to eliminate. Obviously a few chloride ions in the pore water will not break down the passive layer, especially if it is effectively re-establishing itself. [8], [9].
Corrosion Models
The rate of chloride ingress is often approximated to Fick’s law of diffusion. The initial mechanism appears to be suction, especially when the surface is dry. Salt water is rapidly absorbed by dry concrete. The chloride penetration rate is defining the initial concentration as the chloride diffusion is a concentration gradient, not a front [10].
Deterministic corrosion models corrosion may provide a simple and practical means for the structural engineer to estimate the corrosion rate of reinforcing steel based on empirical relationships; these models include some limits related to the random variation of the input model parameters [11].
In this study, Duracrat’s model is chosen as a deterministic model, which integrates parameters relating to the manufacture of concrete and environmental conditions. It is adaptable to a wide range of cement materials; its simplicity allows pairing it with a probabilistic algorithm;
Duracrat’s model gives an expression of chloride ions penetration depth X(t) that predict the initiation of corrosion by: [12], [13].
Where:
Ccrit is the critical chloride ion content
kt is the factor to calculate the diffusion coefficient Kt =0.85
ke is the factor depending on the climatic conditions, ke=1
Drcm is the coefficient of migration of chloride ions (m².s-1), Drcm=4.7.10-12 m².s-1
kc is a parameter taking account of the conditions of curing compound concrete, kc=1
t0 is the expiry considers (year), t0 is the reference period (28 days),
n is a age factor, n = 0.60
Theoretical formulation
Probabilistic Analysis of Reinforced Concrete Corrosion
The randomness effect analysis of the chloride ions migration coefficient (Drcm) and critical chloride ion content (Ccrit) on the reinforced corrosion concentrates on the evaluation of chloride ions penetration depth X(t) from a probabilistic analysis.
The parameters of the lognormal distribution of Ccrit and Drcm are expressed as [14], [15].
| $$\mu_{\ln C_{crit}}=\ln(\mu_{C_{crit}})-\frac12\sigma_{\ln C_{crit}}^2$$ | (5) |
| $$\sigma_{\ln C_{crit}}^2=\ln\left(1+\frac{\sigma_{C_{crit}}^2}{\mu_{C_{crit}}^2}\right)$$ | (6) |
| $${\mathrm\mu}_{{\mathrm{lnD}}_{\mathrm{rem}}}=\ln({\mathrm\mu}_{{\mathrm D}_{\mathrm{rem}}})-\frac12\mathrm\sigma_{{\mathrm{lnD}}_{\mathrm{rem}}}^2$$ | (7) |
| $$\mathrm\sigma_{{\mathrm{lnD}}_{rem}}^2=\ln\left(1+\frac{\mathrm\sigma_{{\mathrm D}_{rem}}^2}{\mathrm\mu_{{\mathrm D}_{rem}}^2}\right)$$ | (8) |
Where () and () are statistics (mean and variance) of Ccrit and Drcm respectively.
Monte Carlo simulations are realized, 10000 independent samples of the parameters Ccrit and Drcm with a log-normal distribution are generated and a deterministic numerical procedure is applied to each individual simulation, providing 10000 values of the chloride ions penetration depth parameters [16].
Finally, time factors statistics (mean, standard deviation and confidence interval) are calculated.
To evolve towards the probabilistic approach, we used the Duracrete deterministic model calculating the chloride ions penetration depth X(t).
The characteristics of the considered medium are shown in Table 1.
Table 1. Characteristics of studied concrete
| Parameters | Value |
| G/S | 3 |
| W/C | 0.5 |
| d [cm] | 3 |
| εc | 0.5 |
| ρw [Kg/m3] | 103 |
| ρc [Kg/m3] | 3150 |
| ρG [Kg/m3] | 2400 |
Where:
G/S is the gravel to sand ratio
W/C is the water to cement ratio
d is the coating
εc is the concrete porosity
ρw, ρc and ρG are the water, cement and gravel densities.
The mean values (μ) and the coefficients of variation (Cv) of the different parameters were estimated respectively from Model Code FIB proposals [14].
μC crit = 1% and CvDrcm varies from 0.1% to 0.5%
μD rcm = 4,7.10-12 m2.s-1 and CvDrcm varies from 0 to 0.04
Results and Discussion
In the present section, we focus on investigating the corrosion process in a heterogeneous media, accounting for the variability of the chloride ions migration coefficient (Drcm) and critical chloride ion content (Ccrit).
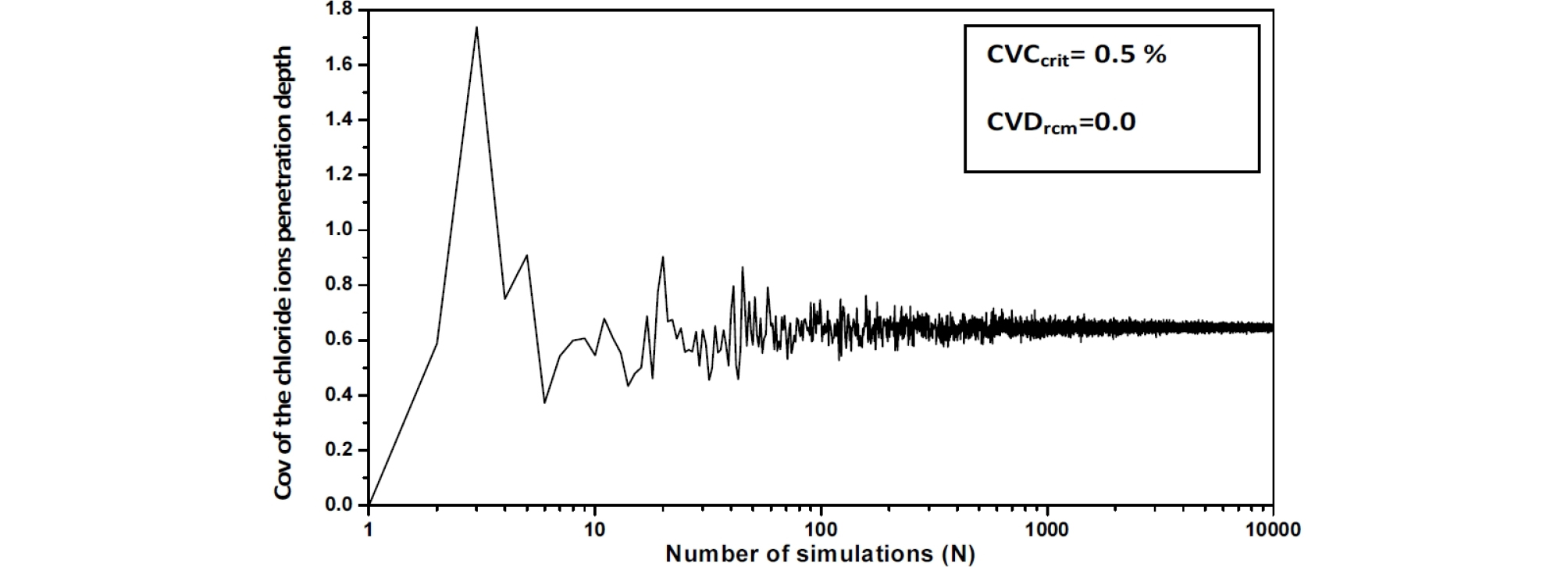
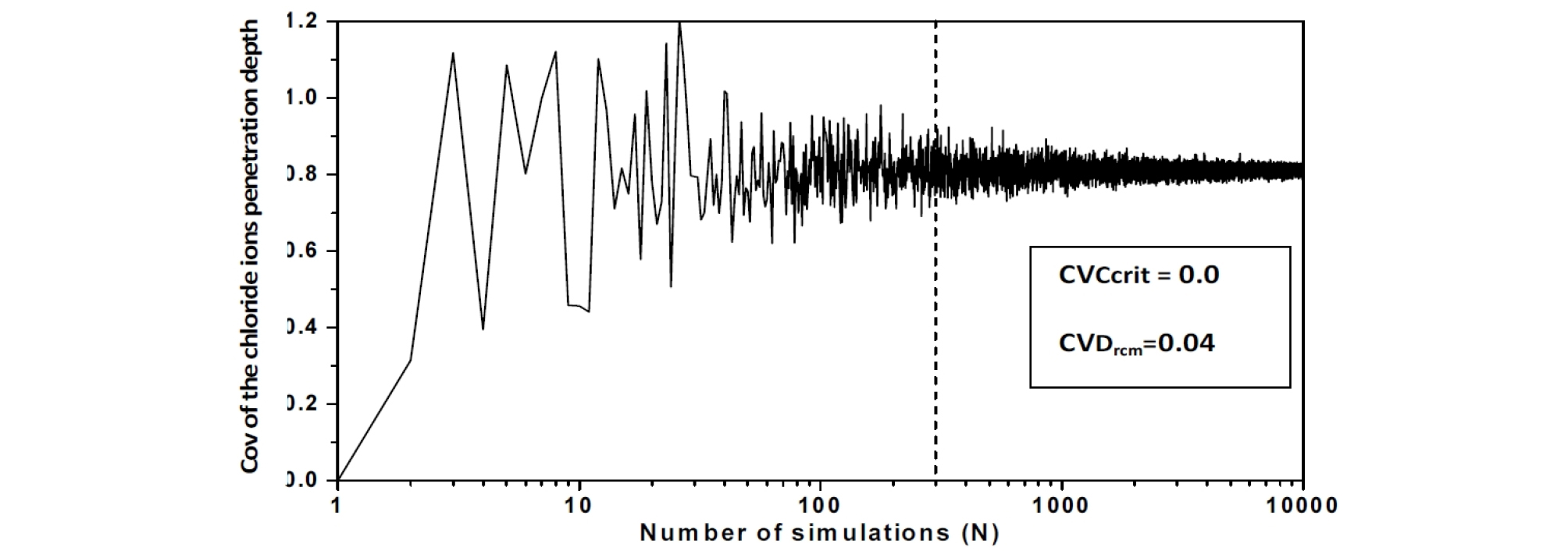
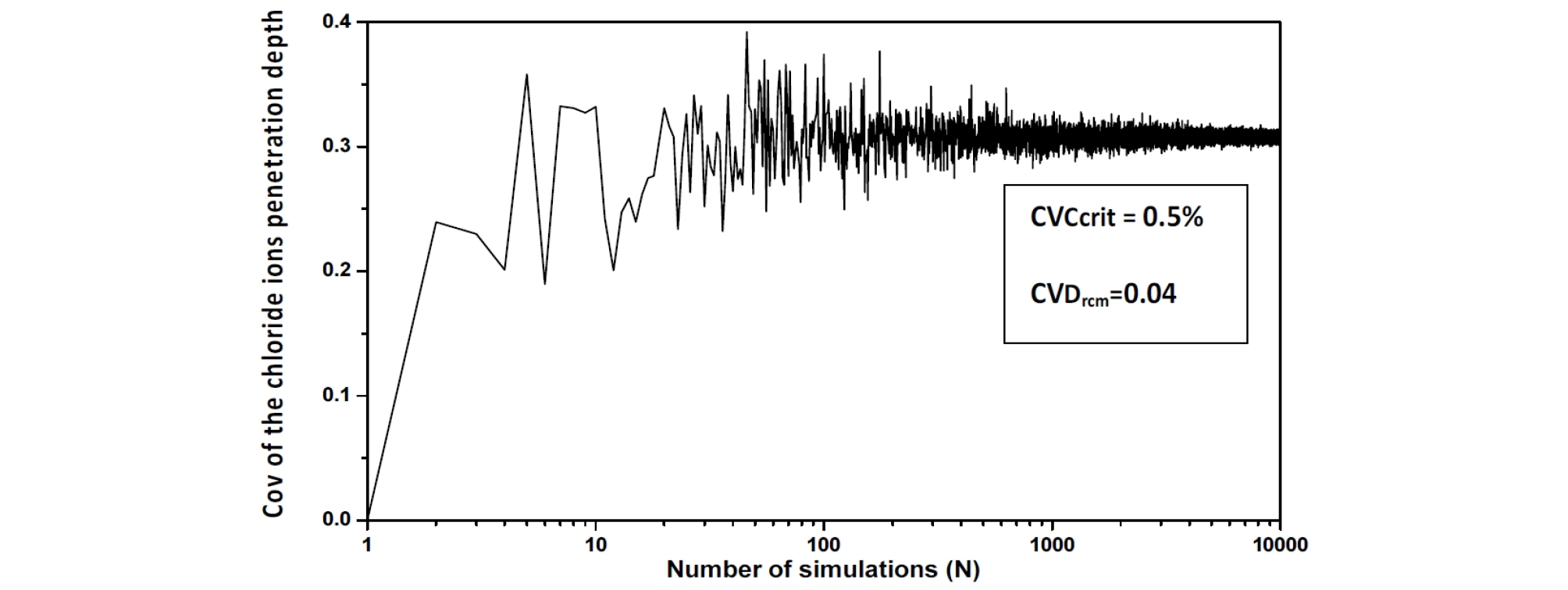
The behavior of the chloride ions penetration depth coefficient of variation versus the realizations number is also investigated. The convergence of the final settlement coefficient of variation versus Ccrit. and Drcm is observed for a number of realizations Nsamp around 400, this number is chosen equal to 10000 [16], (Figure 2, Figure 3 and Figure 4).
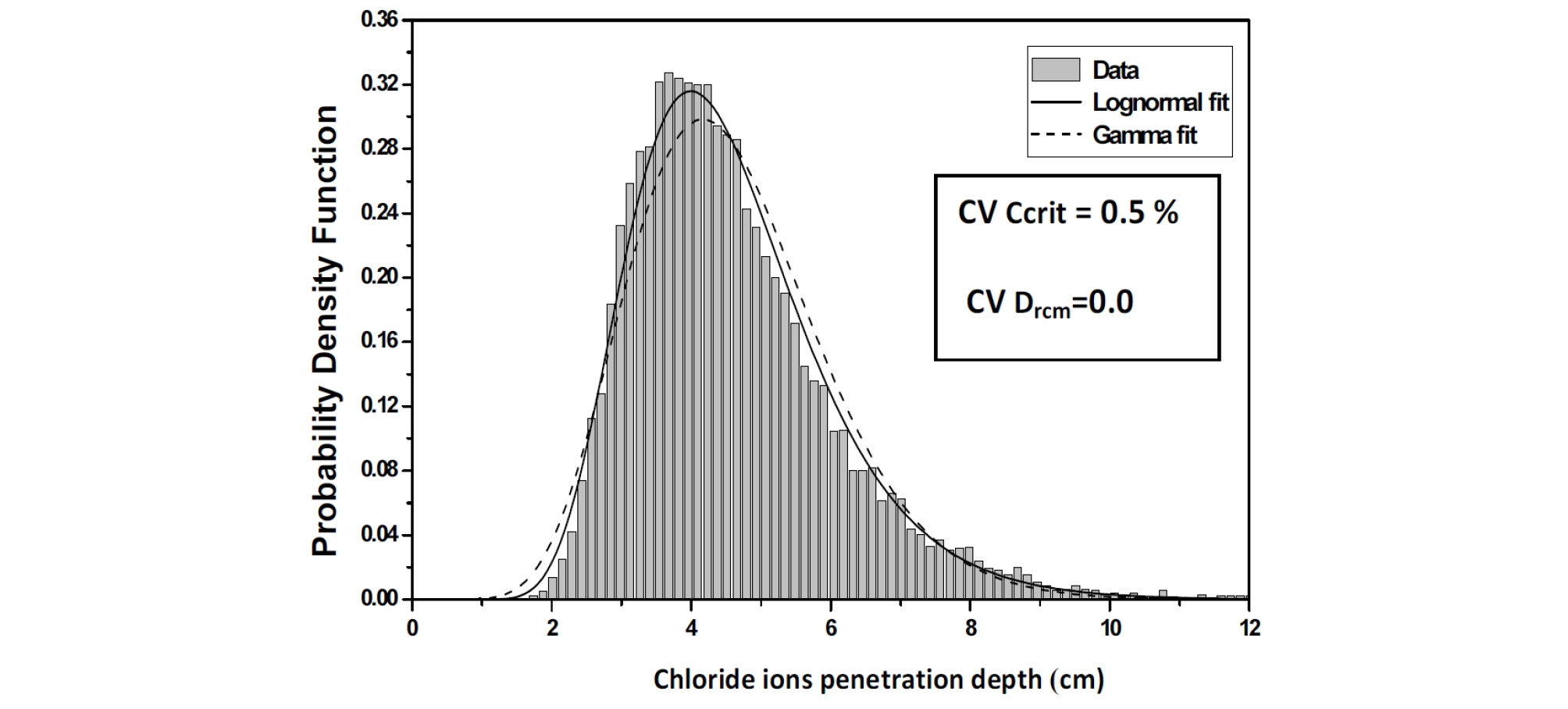
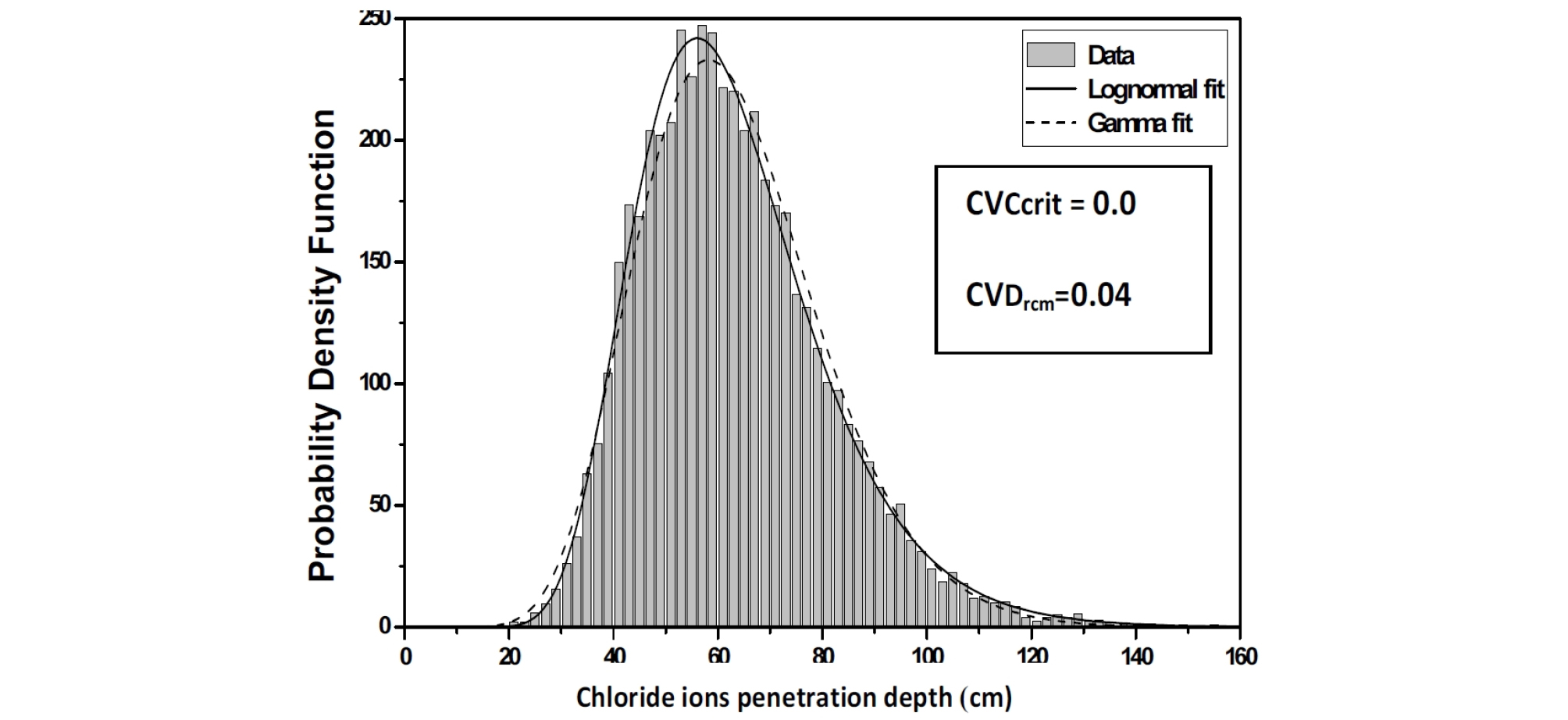
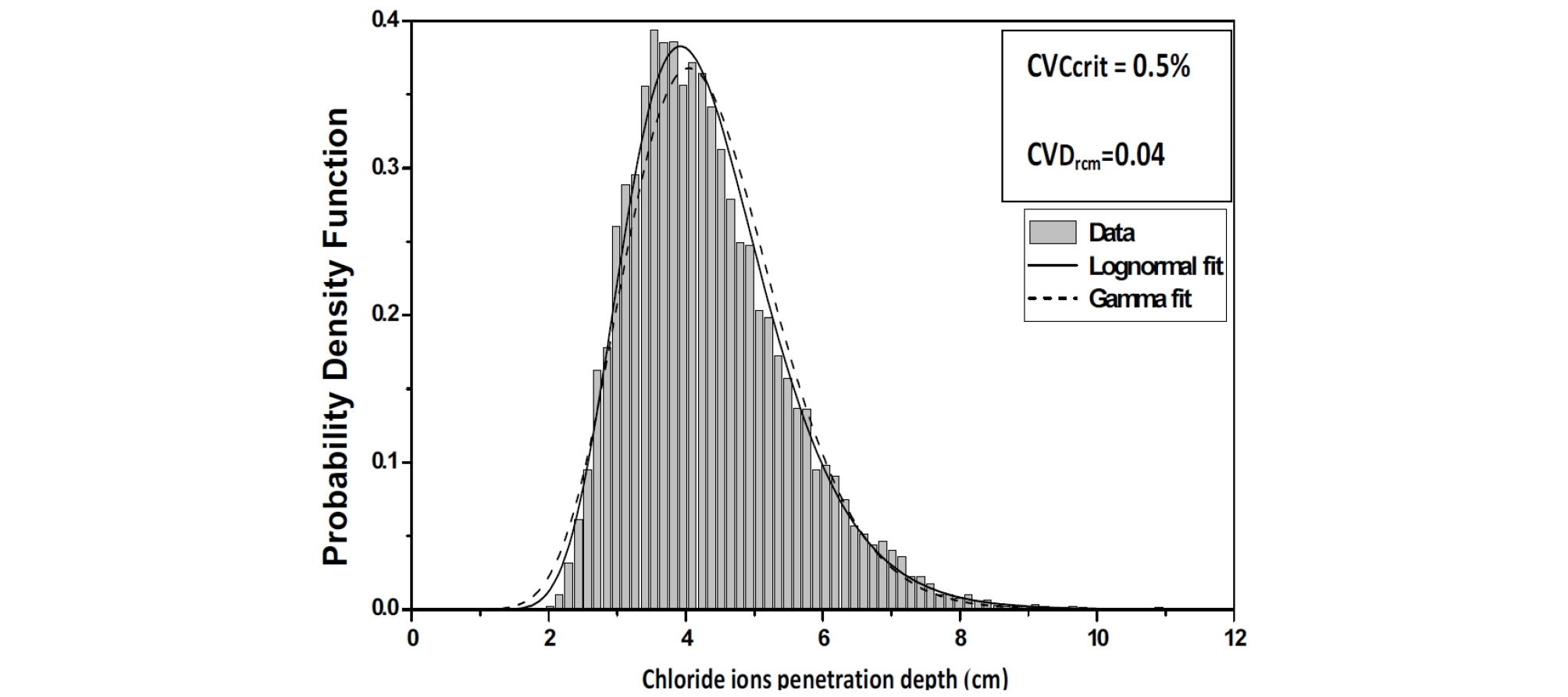
The Chi-Square goodness of fit test is used to evaluate the fit of the assumed chloride ions penetration depth parameters probability distribution, the shape of the corresponding histograms suggests a log-normal distribution, which is suitable for strictly non-negative random variables with large values of coefficient of variation because in this case, simulations with normal distribution can give negative values. (Figure 5, Figure 6 and Figure 7).
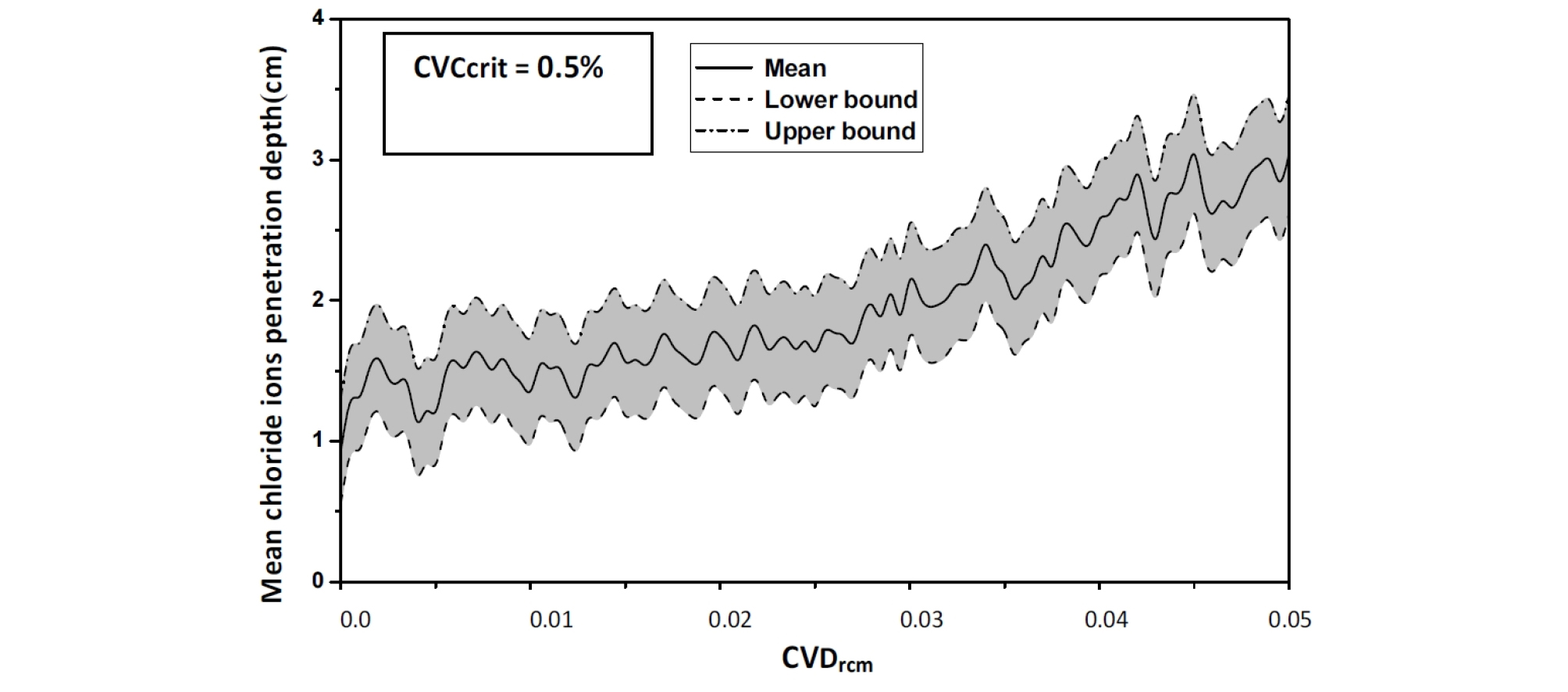
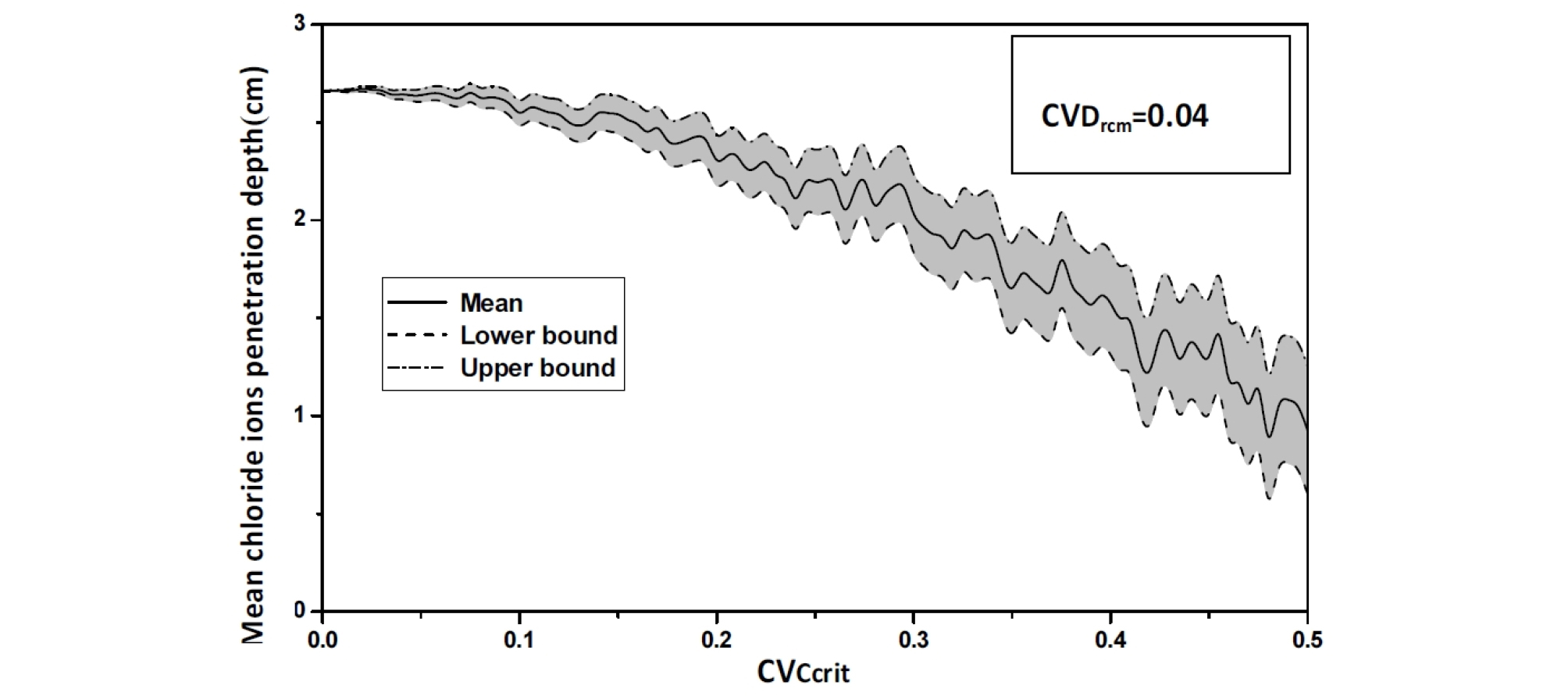
For an assumed log-normal distribution of Ccrit, and from the statistics (mean and standard deviation, as well as the 95% confidence interval) of the chloride ions penetration depth with respect to Critical chloride ion content coefficient of variation, it is shown that the mean value is constant for lower values of CVCritt and CvDrcm, it increases by 1,5%.
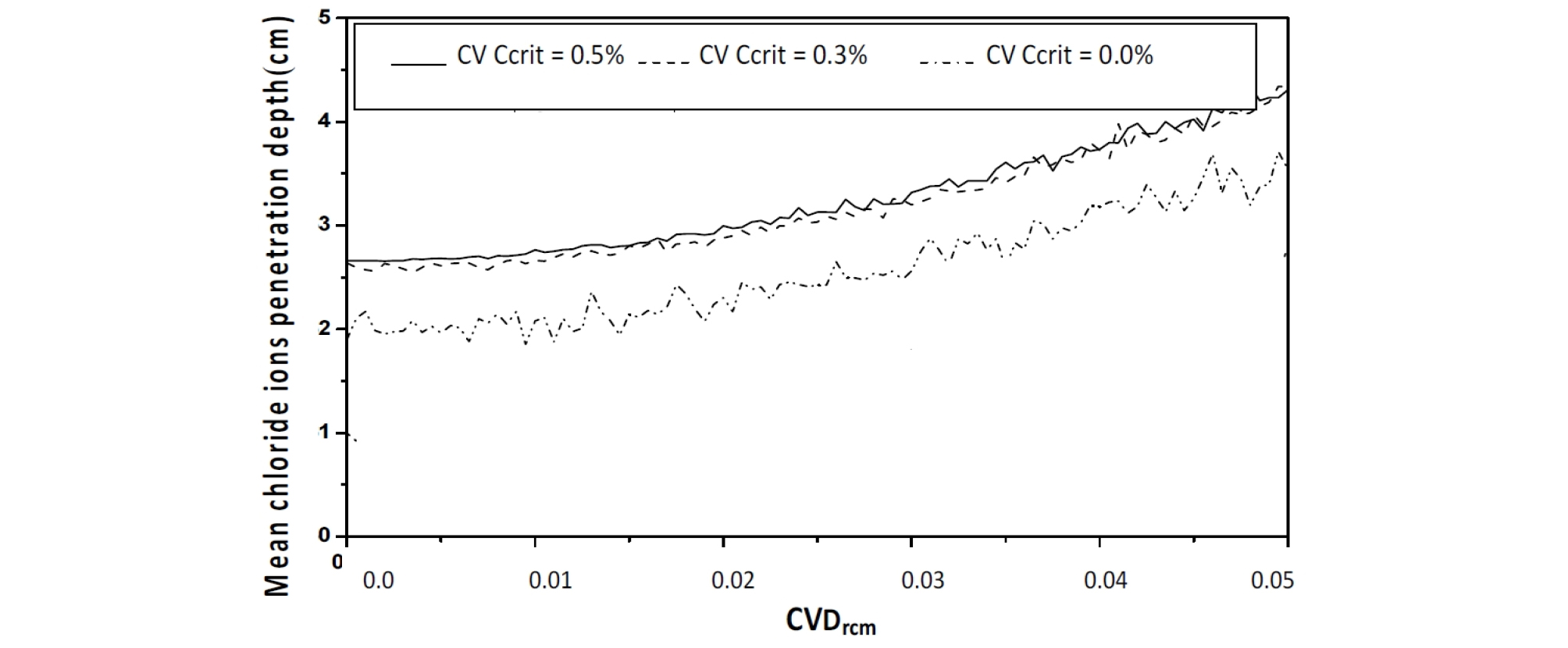
Varying CvDrcm from 0.0 to 0.05, leads to increase in the mean chloride ions penetration depth depth from 2.6 to 4.3 cm (63.38%), see Figure 8.
The penetration of chloride ions within concrete often occurs by diffusion, that is, by the effect of a concentration gradient. For CVCritt= 0.5% and CvDrcm=0.05, the chloride ions move through pores from the surface, where they are present in higher concentrations, to internal zones where their concentration is lower.
On the other hand, chloride ions diffuse only when dissolved in pore water; the diffusion is more effective in saturated than in partially saturated pores. The chloride ions penetration depth depends on the diffusing species, on the characteristics of the concrete and on the environmental conditions. This coefficient can follow variations in the pore structure, or of the external humidity (thus the degree of saturation of pores) or the temperature.
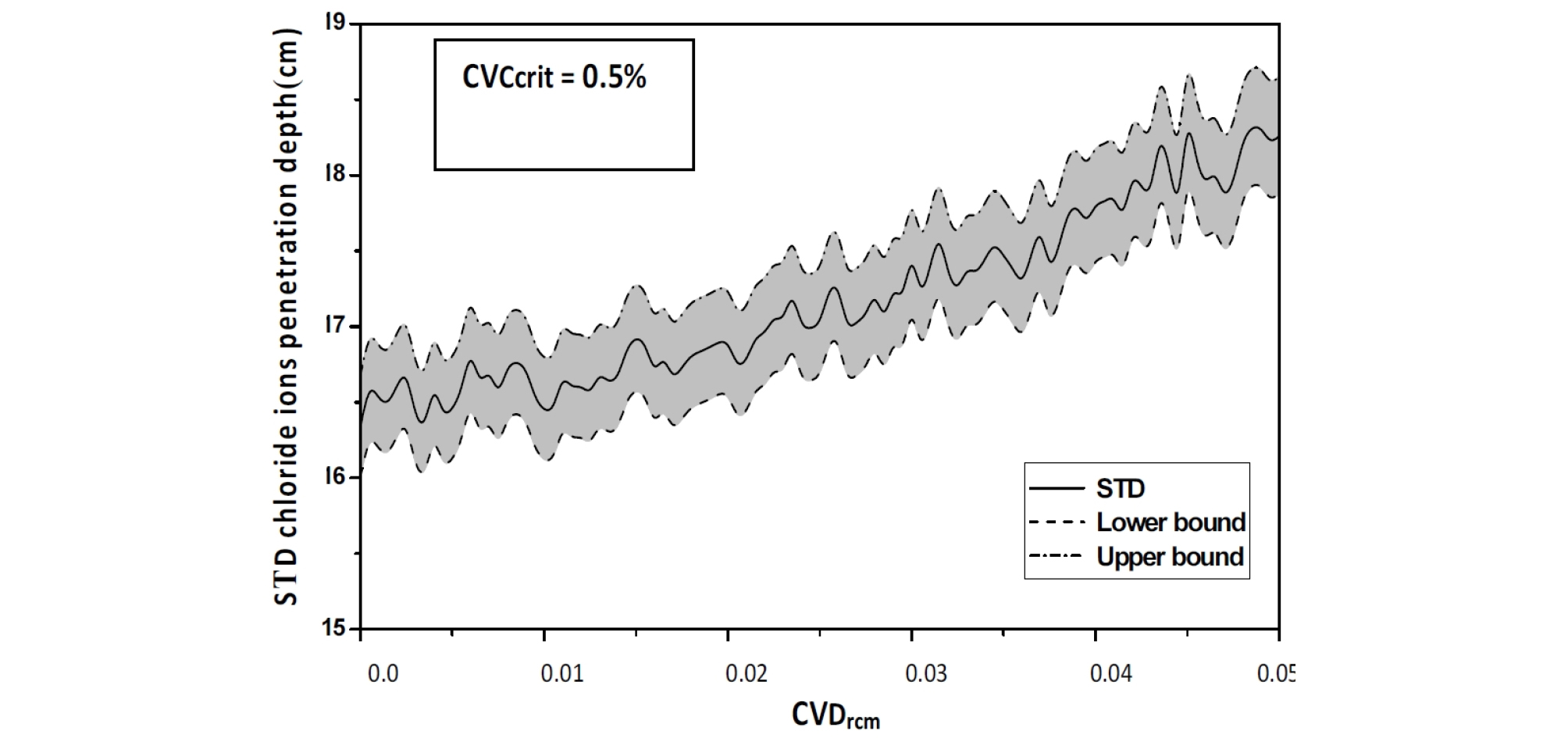
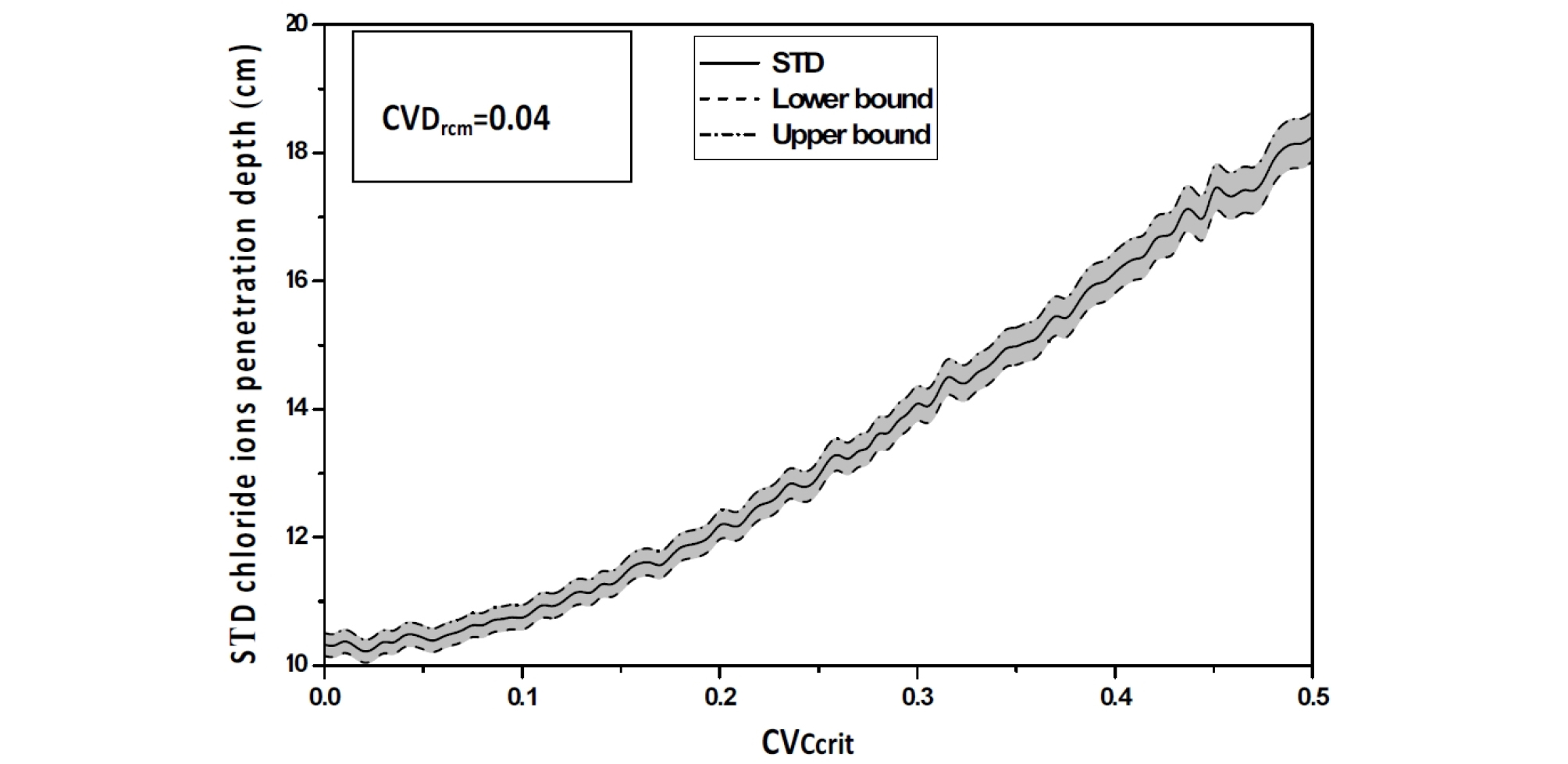
The SDT value of the chloride ions penetration depth increases which indicates that the uncertainty on the Drcm causes a delay in the corrosion process (Figure 9)
The effect of large values of variability of the chloride ions migration coefficient (Drcm) and critical chloride ion content (Ccrit) is preponderant over the small values. Variability of Ccrit causes a delay in the corrosion. For Low chloride ion content values, the diffusion processes of chloride ion to the surface reactive minerals becomes extremely low and the associated reaction mechanisms largely unavailable.
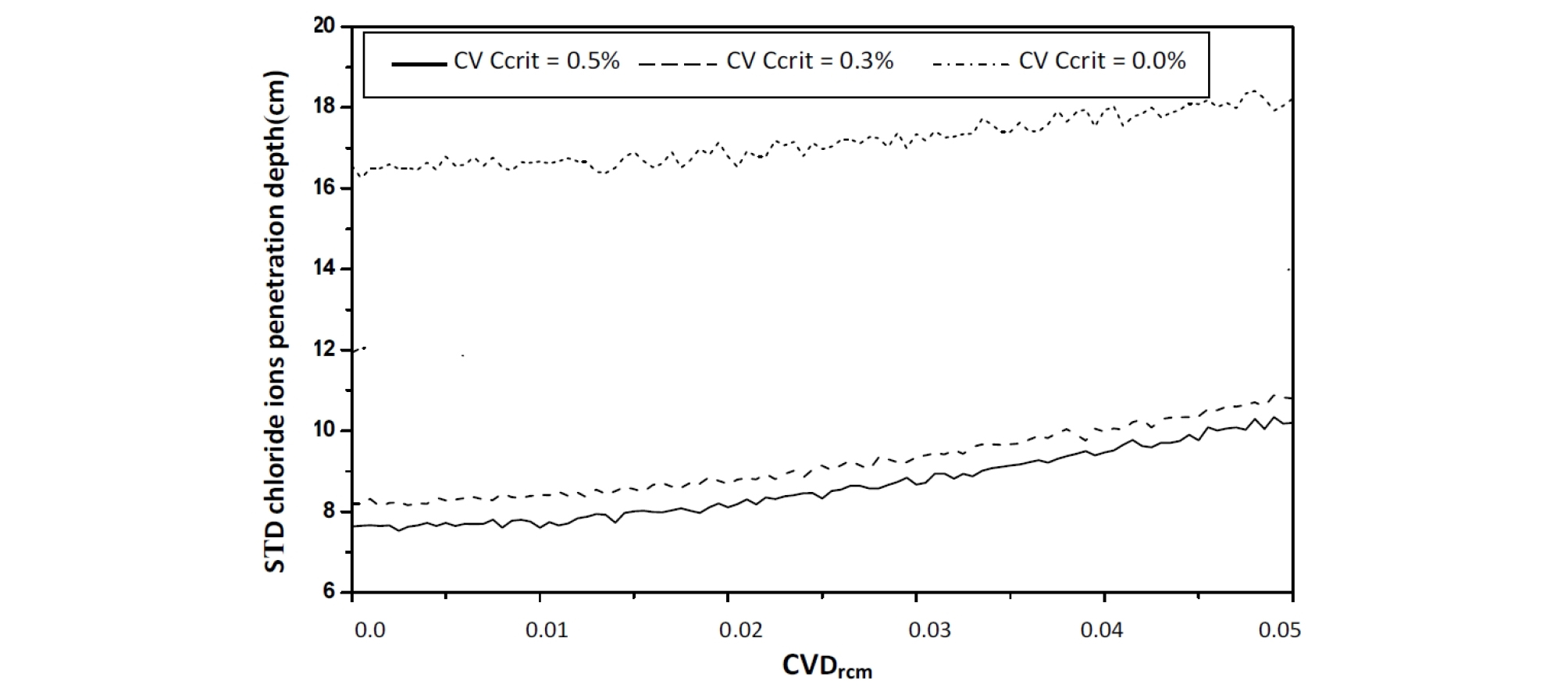
The confidence interval is important, and constant, indicating that chloride ions migration coefficient variability Drcm affects the dispersion of the chloride ions penetration depth, with a weak effect on the mean value, see Figure 10 and Figure 11.
The values of chloride ions penetration depth depends on the pore structure of the concrete and on all the factors that determine it, such as: w/c ratio, compaction, curing, and presence of microcracks. Once the attack begins in chloride-contaminated structures, it can lead in a relatively short time to an unacceptable reduction in the cross section of the reinforcement, even under conditions of normal atmospheric exposure [17] .
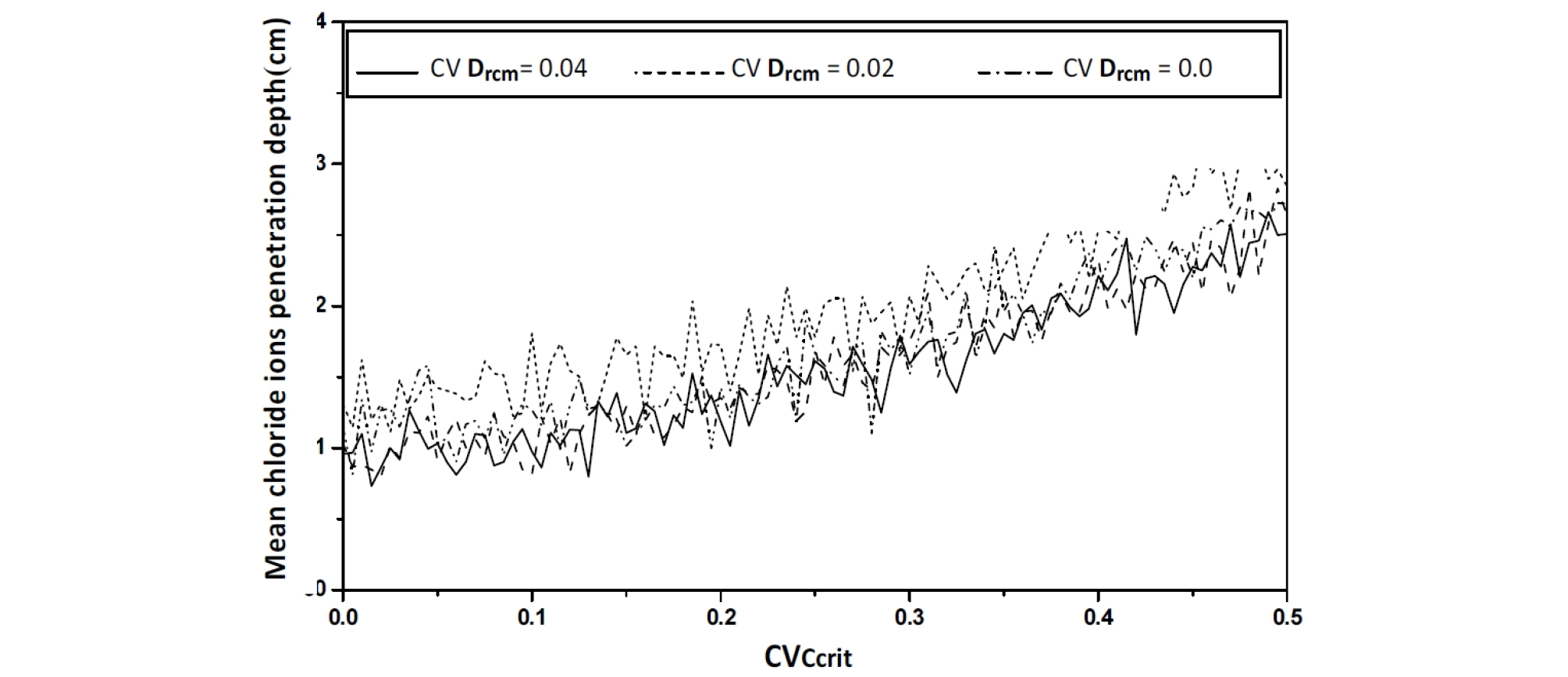
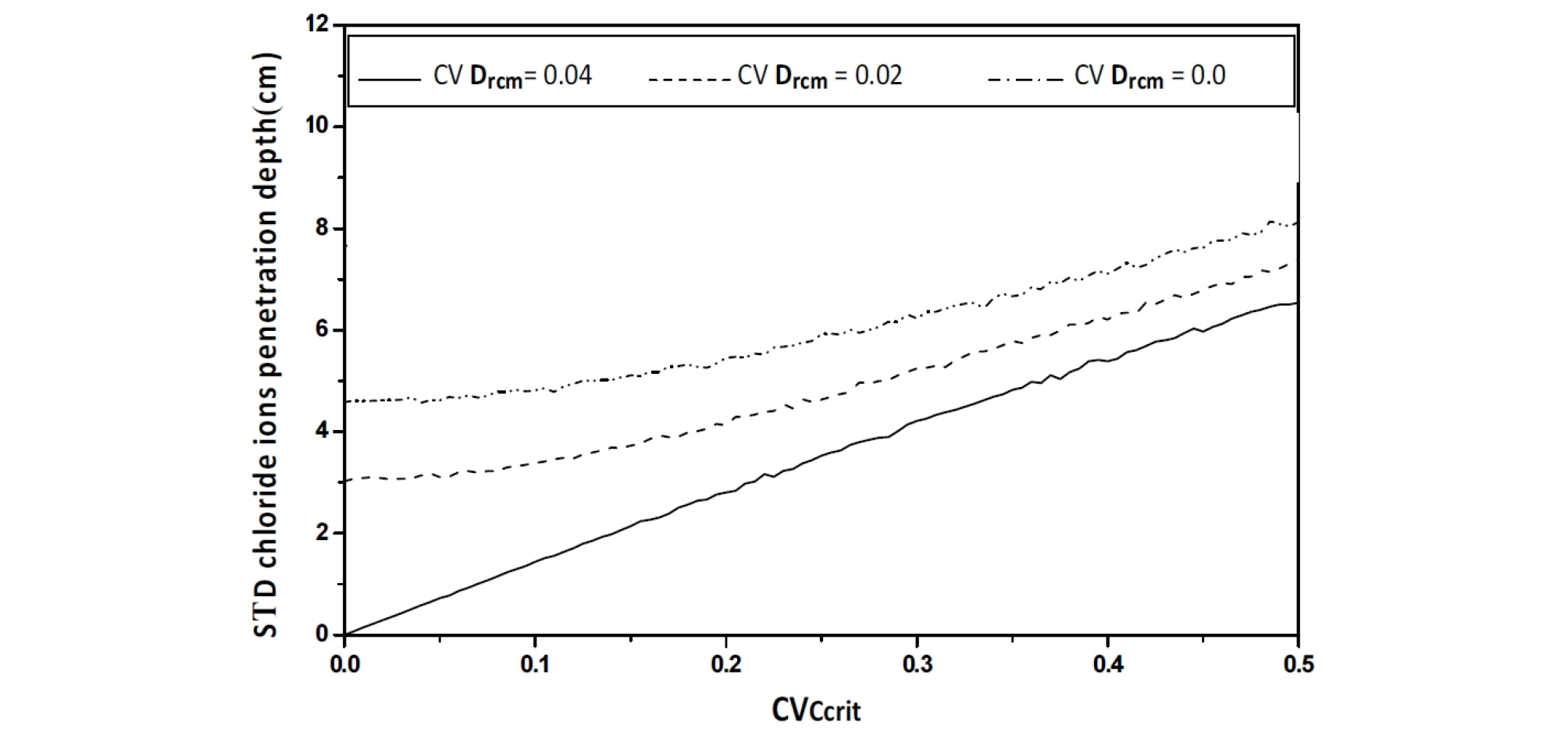
As the coefficient of variation CVCcrit varies from 0 to 0.5%, an increase in the mean value of the chloride ions penetration depth is observed with an important value of its confidence interval, as >As the coefficient of variation CVCcrit varies from 0 to 0.5%, an increase in the mean value of the chloride ions penetration depth is observed with an important value of its confidence interval, as showed in Figure 12, Figure 13 and Figure 14. The standard deviation curve shows a strong increase with linear variation, see Figure 15.
As the coefficient of variation CVDrcm varies from 0 to 0.04, a reduction in the mean and standard deviation values of the chloride ions penetration depth is observed; see Figure 12 and Figure 13.
For CVDrcm= 0.04, the mean and standard deviation values decreases for all values of {\mathrm C}_{{\mathrm V}_{{\mathrm C}_{\mathrm{crit}}}}, the diffuse into concrete can bind to a certain degree with components of the cement matrix, chlorides bind with aluminate phases or are adsorbed on C–S–H; carbon dioxide reacts with alkaline components, in particular Ca(OH)2. The gradual consumption of these compounds modifies the conditions of diffusion and cause a delay in the chloride ions penetration [18], [19].
The confidence interval is important, The standard deviation curve increase with linear variation, see Figure 15, indicating that as for Chloride ions penetration depth , critical chloride ion content (Ccrit) variability affects the dispersion of the penetration depth , with a weak effect on the mean value.
In particular for chloride penetration, often the concentration of the critical chloride ion content (Ccrit) diffusing is taken into consideration and the effects of chemical reactions in concrete are disregarded. In fact, the binding capacity of cement paste is a function of various parameters, such as the critical chloride ion content and the temperature. It will also depend on the chemical composition of the concrete and thus on its variations [20], [21].
Conclusion
The present paper deals with the randomness effect of critical chloride ion content and chloride ions migration coefficient on the corrosion depth.
The critical chloride ions content (Ccrit) variability influences mainly the dispersion of the corrosion depth, but slightly the median value. Indeed, this parameter has an important influence on the interconnection of the porous network, and consequently on the permeability of the concrete and the diffusivity of chloride ions within it.
The values of chloride ions penetration depth depends on the pore structure of the concrete and on all the factors that determine it, such as: w/c ratio, compaction, porosity, curing, and presence of microcracks. More W/C ratio is greater, more the amount of free water that can evaporate is important. By evaporation, the water leaves voids and promotes the diffusion of chlorides through the pore network.
The corrosion kinetic is also affected by pore sizes; the depth of chloride ions penetration tends to increase when the porosity of concrete increases [22]. Once the attack begins in chloride-contaminated structures, it can lead in a relatively short time to an unacceptable reduction in the cross section of the reinforcement, even under conditions of normal atmospheric exposure.
For higher value of CVDrmc, the mean and standard deviation values decreases for all values of CVCcrit ,the diffuse into concrete can bind to a certain degree with components of the cement matrix, chlorides bind with aluminate phases or are adsorbed on C–S–H; carbon dioxide reacts with alkaline components, in particular Ca(OH)2. The gradual consumption of these compounds modifies the conditions of diffusion and cause a delay in the chloride ions penetration [23], [24].