Introduction
Material and Methodology
Materials
Specimens preparation
Test procedure
Results and Discussions
Setting Time
Compressive strength
Heat of hydration
Microstructure of cements pastes
Conclusions
Introduction
For many years, naturally abundant materials such as Pozzolans, limestone and Metakaolin have been shown to be an alternative to Portland cement, as well as some industrial by-products such as fly ash and blast furnace slag [1]. This encouraged the cement industry to use them in their production for economical and ecological purposes. Replacing a quantity of cement with these materials reduces the production costs and the amount of carbon dioxide generated by the production of cement, which represents around 8% of global emissions, and is an important contributor to global warming [2]. The mineral admixtures and other waste product like nano palm oil fuel ash (POFA) were also used to enhance cement properties [3].
The usage of Slag and Pozzolans as cement replacement can slow down the rate of hydration [4, 5, 6]. Thus reducing significantly thermal stresses in mass concrete.
Some studies have shown that the blended cements present an improvement in durability and mechanical performance [3, 4, 5], especially at later ages [2, 4, 7, 8, 9]. The problem is that some mineral additions can lead to a loss of the initial strength [6].
The mechanical and thermal activation have proved to be effective in changing this negative effect [10]. Therefore, based on published results, both methods are employed in this work. The additions used are finely grounded, and the cements are tested at two different curing temperatures in order to highlight the effect of the temperature.
High temperature can significantly increase the degree of hydration at the early ages. One hour at 60°C is equivalent to curing at room temperature for one day [11]. Ezzian and al.[12] studied the effect of cure temperature on cements with different amounts of Pozzolan and noted that, after 1 day, natural pozzolan begins to contribute to the development of mechanical resistance, because of the improvement of its pozzolanic reaction. For a substitution rate of 20% (PZ) and a cure temperature at 40°C, the resistances reach those of ordinary cement. According to Escalante and Sharp [13], the reactivity of slag is also affected positively by the enhancement of temperature. The reaction rate increases linearly with increasing curing temperatures [14].
According to Bougara and al., the replacement rates of 30% and 50% of cement by slag, 40°C temperature is the optimum cure temperature value [15]. Their results demonstrate that the application of thermal activation is very useful when the early strength development is desired. However, for high ultimate strengths, the mechanical activation is more beneficial.
A study of the development of strength on mixes based on blast furnace slag with varying fineness made by Ôner and al [7] concluded that it is not only the fineness of the mix but also of the individual component which govern the choice of the mix composition for a desired strength. The increase of the specific surface improves the strength for mortar containing up to 30% of slag, with their resistances comparable to those of a control mortar [16].
Moreover, the hydration depends on both reagent characteristics and reaction conditions [17]. The mechanism of hydration of the blended cements is more complex, not yet fully understood, hence further research is needed to provide information to increase the knowledge in this field [13]. This work is a contribution to the study of the mechanism of hydration and the strength development of binary and ternary blended cements, based on natural pozzolan and granulated blast furnace slag. This is done by measuring the heat of hydration and monitoring the development of resistances and microstructure.
Material and Methodology
Materials
The materials used in the study with some of their characteristics, are described below.
Cement°(C)
The used cement is a CEM I 42.5 from the Zahana factory (western Algeria).
It contains a low level of limestone; its specific gravity is 3.11 g/cm3 and its Blaine surface area is 3477 cm2/g.
Blast furnace slag
The blast furnace slag comes from the steel complex of Annaba (North Eastern Algeria). Its specific gravity is 2.89 g/cm3. After grinding, it has a Blaine specific surface of 5277 cm2/g. The slag has a glassy structure, allowing it to be used as a supplementary cementitious material.
Pozzolan
The pozzolan used is brick colored from the Bouhamidi deposit in Beni Saf (western Algeria), with a specific gravity of 2.68 g/cm3 and a Blaine specific surface of 9073 cm2/g after grinding. The chemical analysis of the used binder components were carried out at the laboratory of cement plant (Standard methods), and are given in Table 1 together with the Bogue composition of cement.
Table 1. Chemical characteristics of binder components
Specimens preparation
Mortars for compressive strength measurement were prepared by mixing 1350 g of standard sand, 450 g of binder, and constant Water/Binder (W/B) ratio of 0.48. After applying prescribed mixing and molding procedure, mortar specimens are covered with plastic film and cured at room temperature. After 24 hours, the blocks were demoulded and placed in water at a constant temperature of 20°C and 40°C.
Mortars for Langavant test consists of (1080 ± 1) g of standard CEN sand; (360.0 ± 1) g of binder and (180.0 ± 1) g of water.
The pastes for setting test, XRD and MEB were prepared with 500 g of binder and the same W/B ratio of 0.254. The specimens were conserved in the same cure conditions as mortars’.
To identify the specimens, the first letter in each mix label represents the nature of mix (M for Mortar and P for paste) the letter T represents the control specimen (100% of cement). The numbers indicate the percentage of replacement of binder by mass with mineral additions, 0, 1, 2, 3 for 0%, 10%, 20%, 30% respectively. The designation of specimens and mixtures proportions details are shown in Table 2.
Table 2. Designations and mixture proportions
Setting
The setting test using Vicat apparatus was carried out on different pastes mixtures, according to standard EN 196-3 [18]. The initial setting corresponds to the time when the needle penetrates the cement paste at a distance of (4mm ± 1mm) from the base of the mold. The final setting is the time measured from zero time, after which the needle penetrates to 0.5 mm only.
Calorimetry
The effect of mineral additions on the hydration kinetics of mortars is determined using semi-adiabatic calorimetry test also known as the Langavant method. It is designed to continuously measure the heat release by cements during the first days of hydration. The mixes were prepared and tested according to EN 196-9 standard [19].
Compressive Strength
The compressive strengths of mortars were determined on prismatic specimens (4 × 4 × 160 mm) according to EN 196-1 [20] standard. The results are the average over six test pieces.
Scanning Electron Microscopy
The fracture surface of some pastes was observed by using SEM, model (Jeol IT300), equipped with EDS, at 7, 28 and 90 days of hydration. Small fragments were taking from the center of specimens, and then were adhered on a two side adhesive black tape. All samples were also coated with carbon before using SEM.
X-ray diffraction (XRD)
Bruker D8 powder diffractometer , with Cu Kα radiation, was used on PT, P20 and P23 specimen. The pastes were reduced to powder (particle size < 80 μm), at 28 days of hydration and cure at temperature of 20°C and 40°C.
Results and Discussions
Setting Time
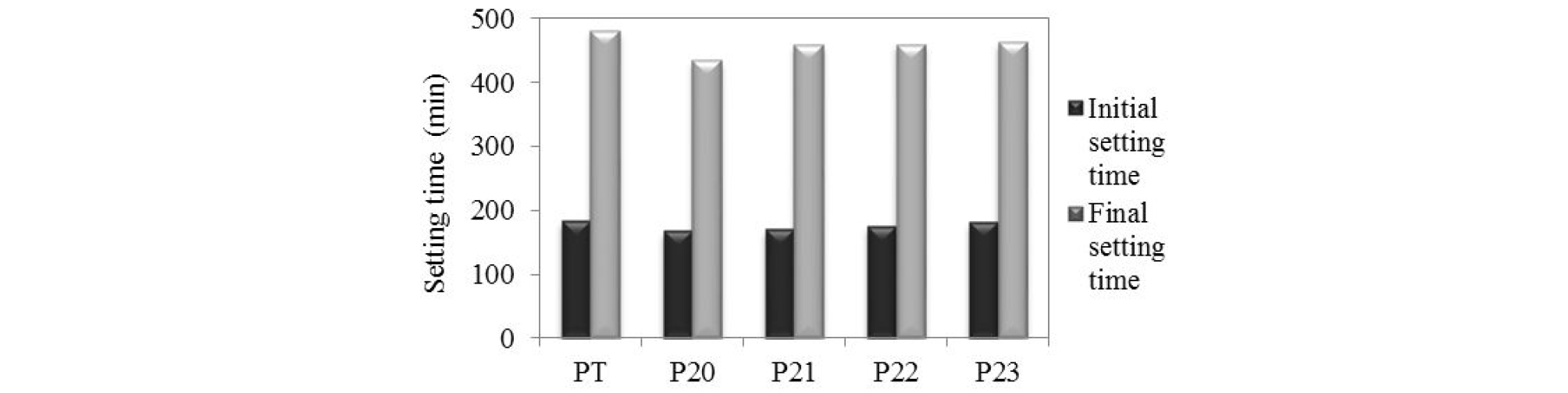
The initial and final setting times of different specimens are shown in Figure 1. The addition of finely ground pozzolan and blast furnace slag resulted in a small reduction in the initial setting time of cements, compared to the control. The presence of fine particles accelerated the hydration of the components and contributed to the increase of the viscosity of mixtures.
The final setting times in the ternary mixtures M22 and M23 are close to the control. The hydration kinetics of the binder slows down, proportionally to the increase of the amount of slag [21].
When cement is replaced by additions, the amount of C3S and C2S in the binder is reduced, resulting in the formation of small amount of calcium silicate hydrates (C-S-H) crystals responsible for the hardening phenomenon [15].
XRD analyses
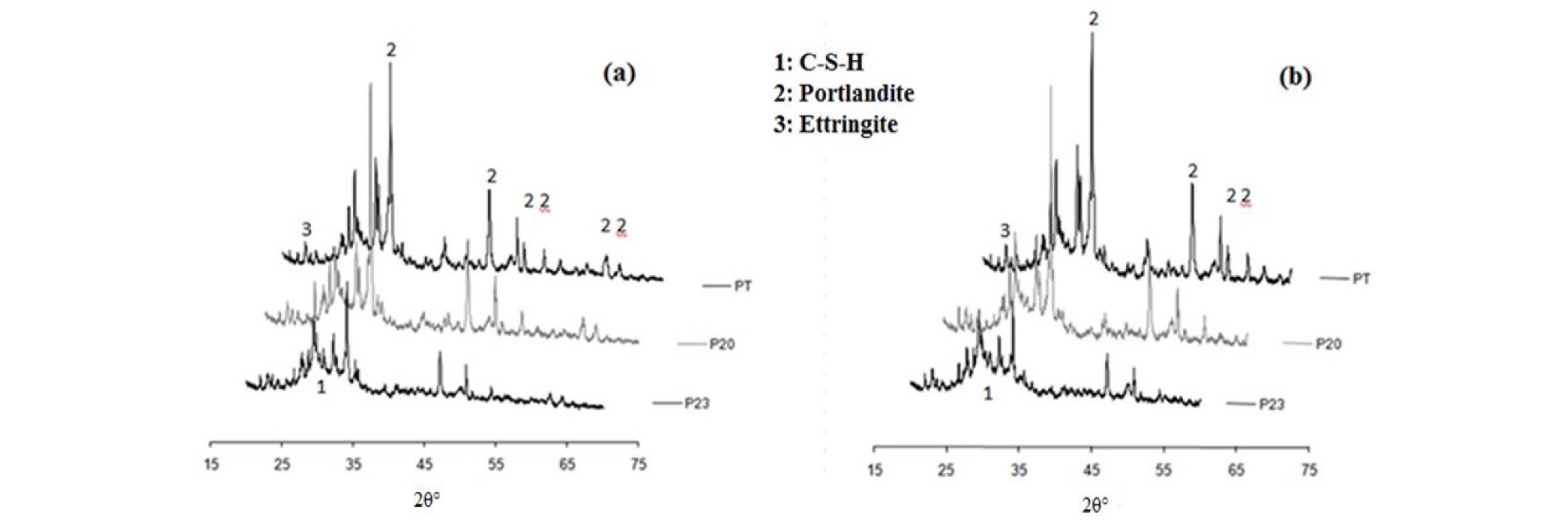
The XRD patterns of PT, P20, P23 mixes at 28 days of hydration are geven in Figure 2.
The pozzolanic reaction fixes the portlandite produced by the cement hydration to form additional C-S-H. It is highlighted in Figure 2(a), which shows a decrease in the intensity of the peaks of portlandite for pastes cured in water at 20°C and an increase in the halo of C-S-H centered at a reticular distance of 3.06 Å, in paste with additions, compared to the control at 28 days of hydration.
Moreover, Figure 2(b) shows that the fixation of portlandite becomes greater in the ternary mixture. This is indicated by the shorter length of Portlandite peaks compared to PT and P20. The halo corresponding to the formation of C-S-H is greater in pastes with additions, cured at 40°C than those cured at 20°C. The increase in curing temperature accelerated the hydration reactions and improved the reactivity of the mineral additions [12, 13].
Compressive strength
The compression test was carried out on MT M20 M21 M22 and M23 mortars at the short, medium and long term, in order to define the effect of the additions and their substitution rates on the compressive strengths. The cure temperature has been increased in order to improve the reactivity of the additions and to enhance the strength at early age.
Compressive strength of mortars cured at 20°C.
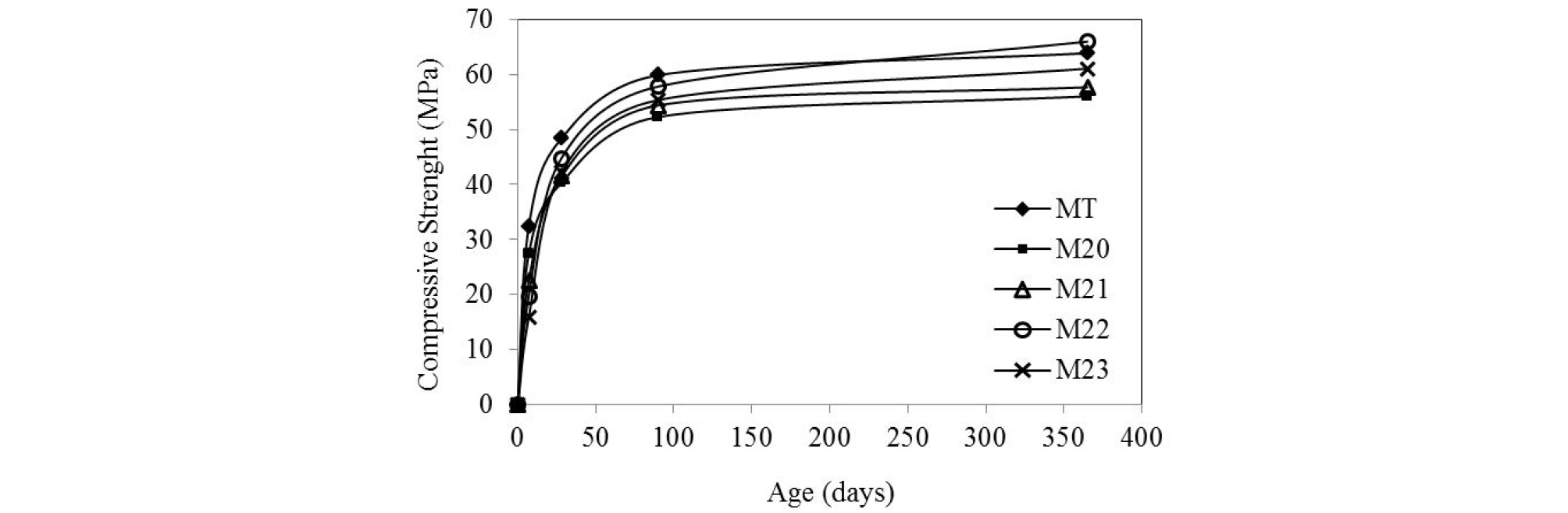
The compressive strengths of mortars at different ages and at 20°C cure temperature are plotted in Figure 3. At an early stage, the M20 mortar with a 20% substitution rate of pozzolan has a lower compressive strength than that of the control (32 MPa for the control and 27 MPa for the M20 mortar at 7 days). In the case of mortars M21, M22 and M23, the resistance decreases by 30%, 38% and 50% respectively, compared to the control.
The compressive strength loss is proportional to the substitution rate of the cement by the additions, as a consequence of the reduction of the active part of the cement in the mix [12, 15, 22]. Long term strength of control mortar and M20 mortar tends to stabilize but continue to increase for mortars with slag. This result is in good agreement with Baron et al [23]. Who found that the pozzolanic reaction is practically constant between 2 and 28 days. However, after 28 days, the pozzolanic activity accelerates, improving the mechanical resistance and the durability.
The maximum compressive strength after one year is 66 MPa for the M22 mortar and 64 MPa for the control.
As reported in the literature, the development of mechanical strengths in cement composite is low in the short term, but in the long-term, strengths exceed those of ordinary cement [24, 14, 22, 23]. The increase in strength of mortars with additions is attributed to refining pores and to the increase of the amount of C-S-H in the cement matrix [25], confirmed by XRD.
Compressive strength of mortars cured at 40°C.
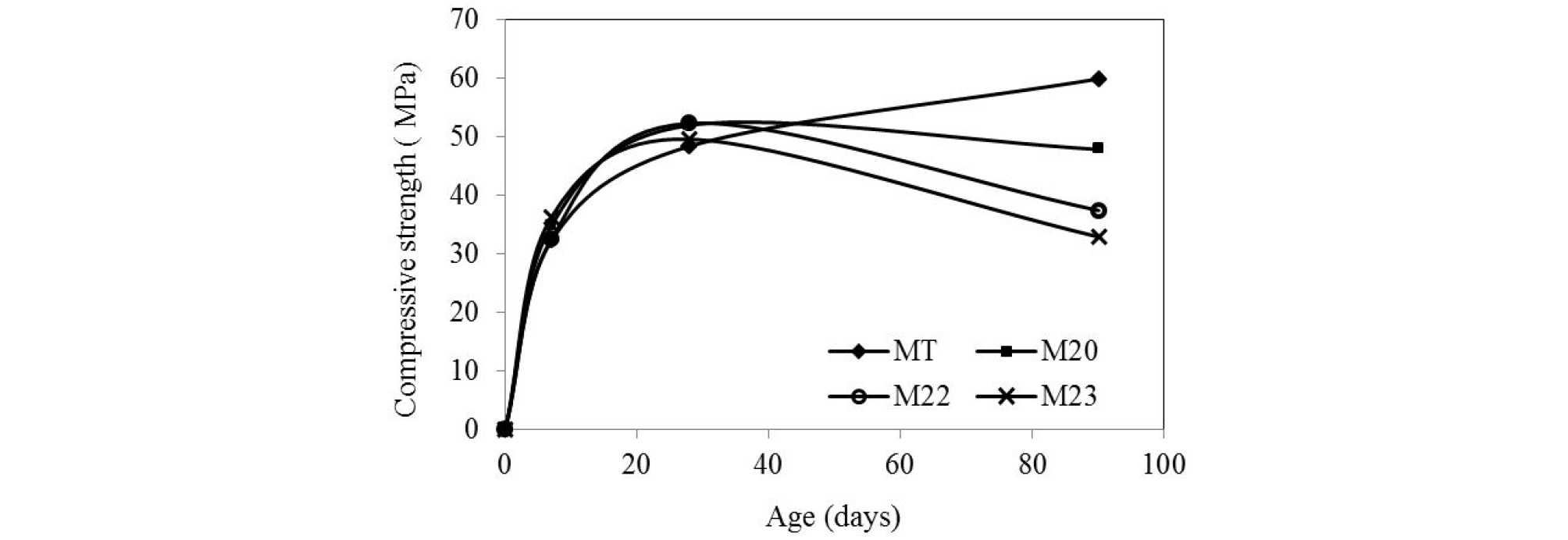
Figure 4 represents the evolution of compression strength of mortars cured in water at 40°C. It indicates a rapid increase of compressive strength at short and medium term. For M20 mortar it’s enhanced by 28% compared to MT at 7 days. The compressive strength of the ternary mortars M22 and M23 exceeds the MT at 28 days.
After 90 days in water at 40°C, there is a drop in resistance for all mortars. The compressive strength of mortars M20, M22 and M23 are respectively 20%, 42% and 45% below that of control, cured at standard conditions. A rise in curing temperature during the early stages of hydration improves the development of strength but has adverse effects in the long-term [11, 14, 26, 27].
The positive effect of temperature on compressive strength of mortars is significant on blended cement. The mortars cured at 40°C have a gain of strength of 65% and 130% for the mortars M22 and M23 respectively at 7 days, compared to M22 and M23 cured at 20°C. The strength of M23 exceeds the control mortar at 28 days. The improvement in compressive strength can be attributed to the beneficial effect of the increase in the reactivity of the additions by the cure temperature [12, 11, 14].
Heat of hydration
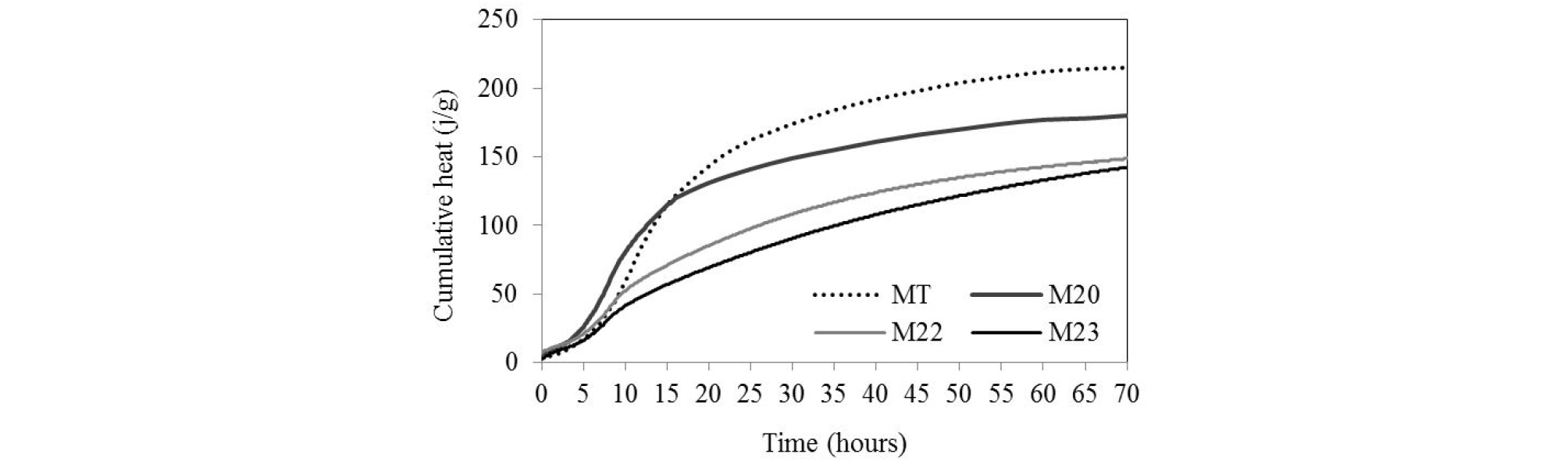
The total heat released by hydration over time is shows in Figure 5. In the initial time of hydration, the heat released by the blended cements was greater than that of the control, especially for M20 mortar. The amount of heat released exceeds that of the control and decreases only after 15 hours of hydration. This may explain the acceleration of the setting time in the mixture with 20% of pozzolan. The fine portions contributed more to the total early heat evolved than coarse portions [28]. In the ternary mixtures, the heat released over time is clearly lower than the control and decreases proportionally to the slag rate.
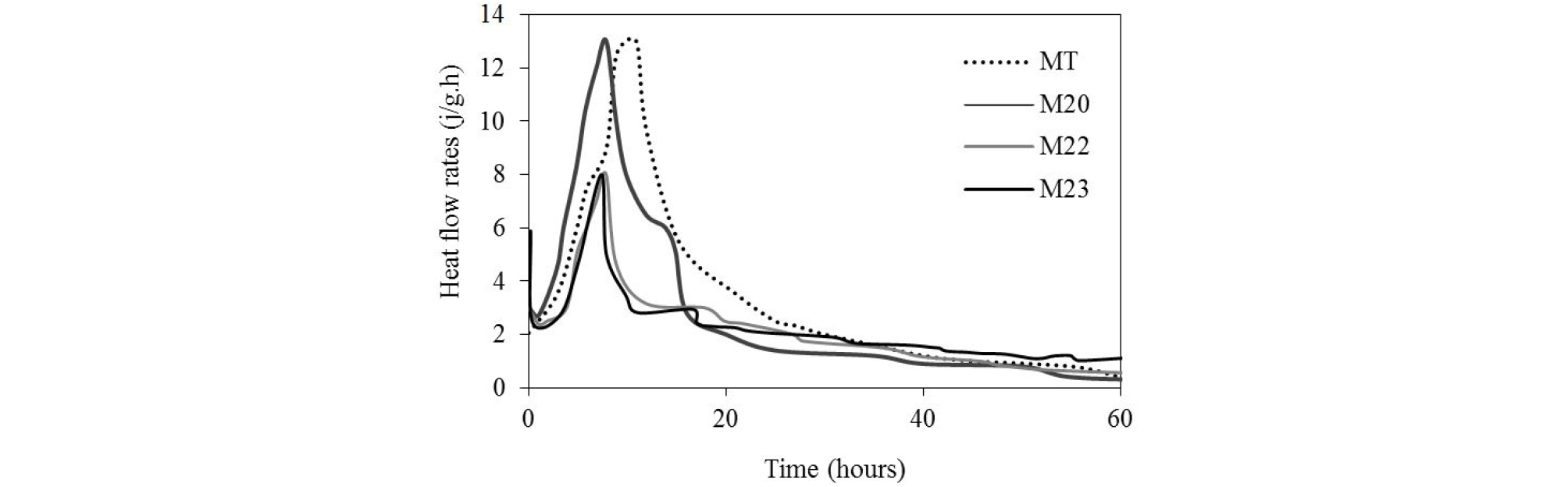
In Figure 6, the change in the rate of the heat flow during the hydration of MT, M20, M22 and M23 mortars is plotted. The hydration process can be divided into five phases, characterized by three maximum peaks [29, 30].
The first hydration peak of the initial period is characterized by a significant heat release due to the hydration of C3A with gypsum and the dissolution of C3S [30]. The peak is not visible on the curves, because the measurements were made after its occurrence.
The second period is the dormant period, during which the chemical reactions and the heat of hydration rate are very low [30]. This period was shortened by a rapid resumption of hydration reactions for M20 mix compare to the control.
The small amount of pozzolan incorporation enhances the short term hydration of the cement [28]. The appearance of the second peak of hydration characterized the acceleration period. The curves show a similar increase in the height of the hydration peaks of M20 and the control mix. The intensity of the second hydration peak decreases considerably in the ternary mortars.
After it reaches its maximum value, the second peak is followed by a deceleration period during which the appearance of a third peak (in the form of a small hump) in the plots of the mortars with additions. This peak is often related to the transformation of ettringite in to monosulfoaluminate, after gypsum exhaustion, and can be due to the pozzolanic reaction [30]. After this, the hydration reactions continue with a very low level of heat during the steady state.
The acceleration of the hydration reactions of the blended cement M20 is probably due to the fineness of pozzolan particles, which offer additional sites of nucleation and precipitation of cement hydrates products (filler effect) more than its reactivity to calcium hydroxide (pozzolanic effect) [31]. The latent property of the slag contributed to regulate the kinetics of hydration and reduced the amount of heat released [3, 32].
Microstructure of cements pastes
The study of micro-analysis is to better understand the physical and chemical behavior of mortars, through the observation of the microstructure evolution of cement matrix. For this purpose, observations using the scanning electron microscope are made on PT, P20, and P23 pastes, prepared with the same proportions used to prepare the mortars MT, M20 and M23. Samples are also kept under the similar cure conditions. The same magnification for images of fractures has been adopted in order to compare between the characteristics of the different specimens.
Pastes cure in water at 20°C.
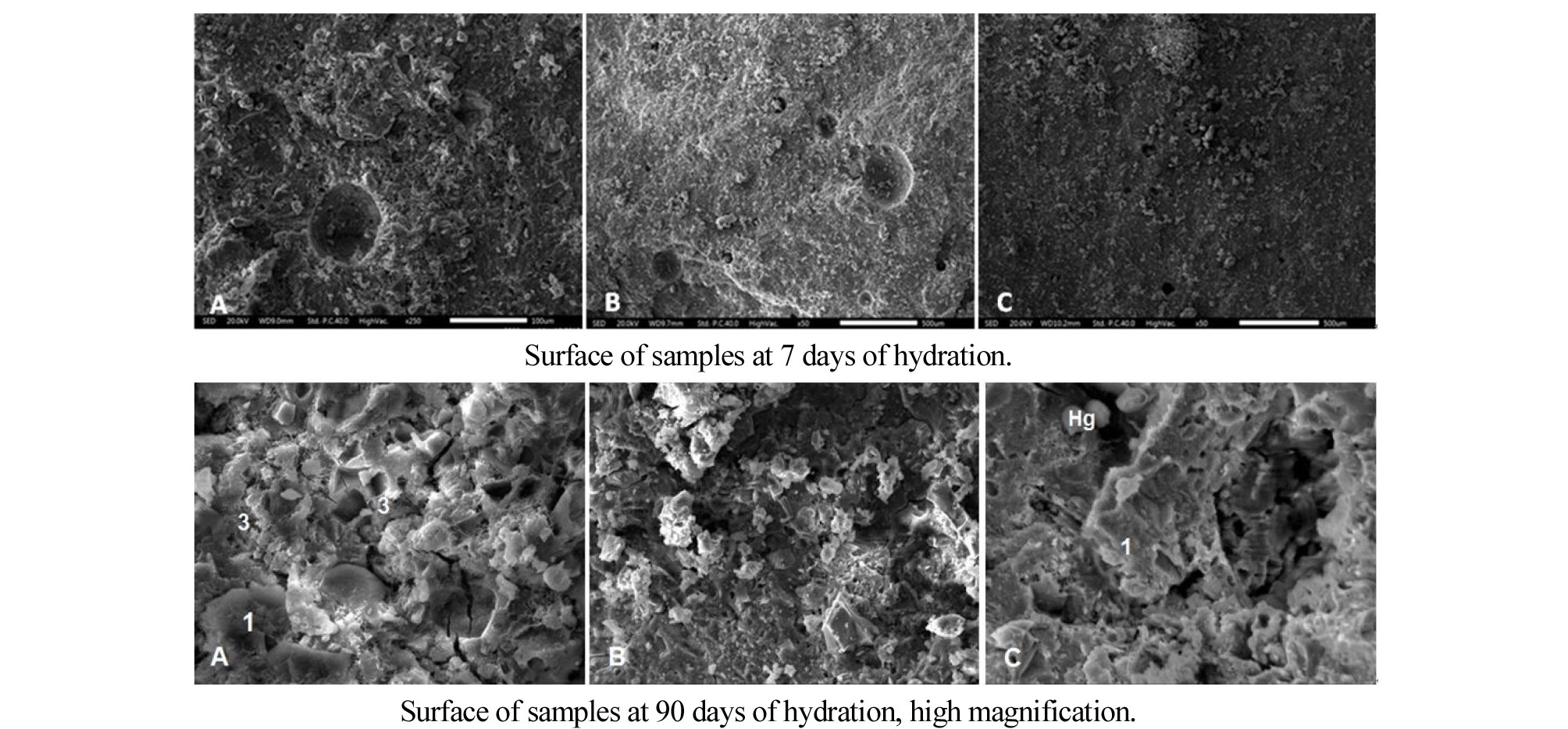
After 7 days of hydration, the micrographs of pastes cured in water at 20°C are shown in Figure 7. The P20 and P23 mixes shown in Figure 6(B) and 6(C) respectively shown more compact structure compared to the control Figure 6(A). The addition of finely ground pozzolans influences the porous structure, because of the pozzolanic reaction, and its filler effect, which results in more homogeneous hydrates with a finer and denser structure [23].
Also, it can be seen that for all mixes there are voids left behind by the entrained air bubbles; however it is greater in the P20 paste. This is in agreement with observations from Belas Belaribi and al [33], who indicate that the pozzolan tends to increase the occluded air content. The micrograph of the ternary mixture P23 Figure 7(C) shows a few voids lefted by the entrained air bubbles on the fracture compared to PT and P20. Therefore, it seems that the addition of slag has reduced the porosity due to the occluded air.
In figure 7 at 90 days, the microstructure of mixtures is different. The micrograph of PT shows the presence of ettringite (3) recognized by its morphology in the shape of needles which is mixed with C-S-H gel (1). The presence of Ettringite has been confirmed by EDS and XRD. Cracks pass through the cement matrix and form part of the porosity. The micrographs of P20 and P23 show no trace of microcracks. There is Hadley grain (Hg), filled with ettringite, visible in P23 paste.
Pastes cure in water at 40°C.
In agreement with [34], the fractures surface of specimens P20 and P23 as shown in Figure 7 and Figure 8, show greater compactness due to the acceleration of the hydration reactions observed with XRD. The microscopy and porosity seem different from that of the samples under standard conditions (Figure 6).
The enlargement of the encircled area in Figure 8 (G) shows the formation of hydration products in the voids, filling the space and changing their structure. There is C-S-H gel (1) detected by XRD in all specimens; Alveolar C-S-H (2) [35], and ettringite in P20. The Portlandite (5), is characterized by its hexagonal plate shape, as shown in Figure 9(J).
Observation of the microstructure suggests that the decrease in the strength of mortars, cured at high temperature, can be attributed to the presence of unreacted cements (4) [12]. During a long period of heating, the thickness of C-S-H around the cement particle increases and densifies, interrupting the subsequent hydration of these cement particles [36].
The fall of the strength can also be due to the formation of micro-cracks, observed in fractures, under the effect of the enhancement of cure temperature [37]. The presence of Portlandite shown in Figure 9(J) indicates that the pozzolanic reaction is much slower in the high slag blends [6], despite the thermal activation.
Conclusions
The observation of the microstructure and the tests results obtained on the pastes and mortars based on finely ground mineral additions, under different curing conditions, leads to the following conclusions:
The finely ground natural pozzolan addition accelerates the cement hydration reaction in the short term, increases the heat release and reduces the setting time. The GBFS contributes to regulate those effects. This shows that a good choice of materials and proportion can improve the behavior of the final product.
The XRD analysis showed a greater amount of C-S-H in mixes with mineral additions. This quantity is more significant when the temperature increases.
Mechanical activation by high grinding additions didn’t improve the early age strengths but, in the long term, the resistance of the mortar M22 was comparable to that of the control. The use of such a mixture leads to a saving of about 40% of cement with a comparable reduction rate of CO2 emissions.
The thermal activation has been more beneficial to early and medium age strength, especially for cements with mineral additions. The M20 mortar gives the best mechanical resistance at 7 and 28 days, and the resistances of M23 mortar is doubled at day 7 of hydration, compared to the same mortars immersed in water at 20°C. For precast concrete cured in controlled environment, the thermal activation can be recommended when the blended cements are used.
It seems that in addition to the degree of cure temperature, it is necessary to take into account the duration and the age at which the heat treatment must be applied. For this case, the curing time at 40°C should not exceed 28 days.
The addition of slag contributed to reducing the porosity due to the occluded air. The development of the porosity and the nature of the hydrate formed are clearly influenced by the nature of mineral additions and the cure temperature.